Projects
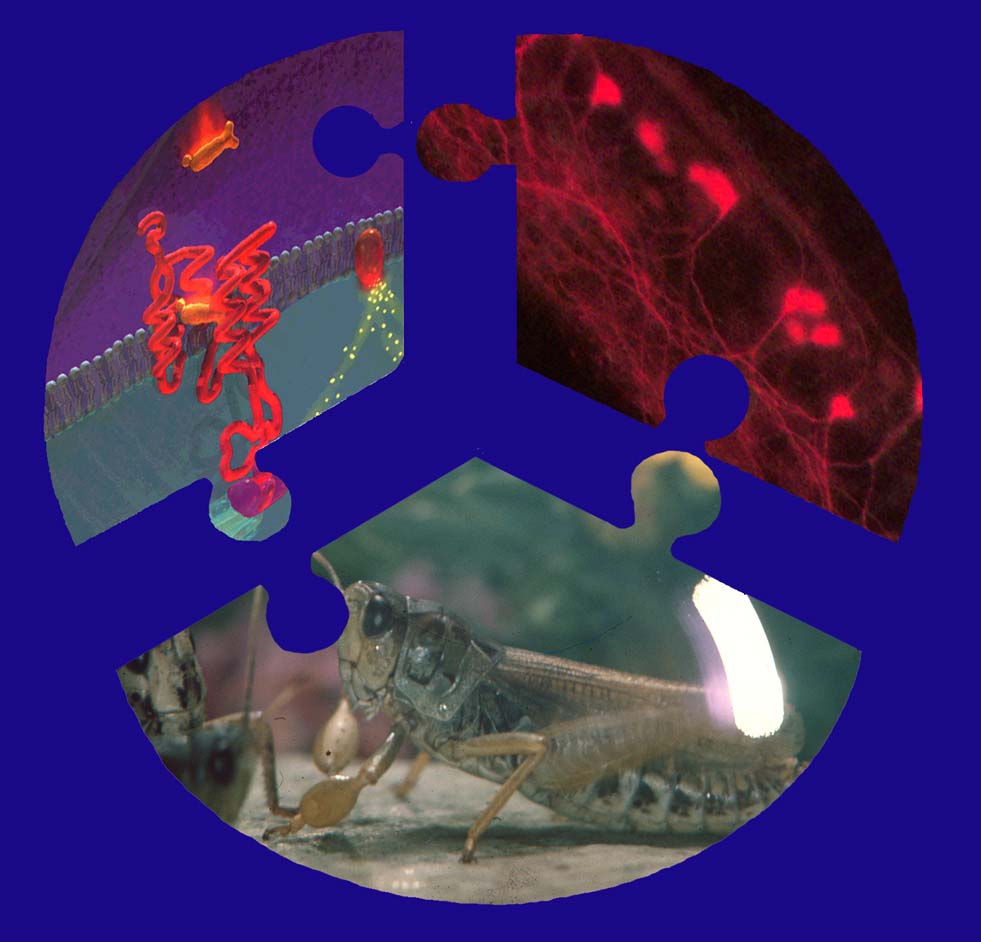
Presence and function of erythropoietin (Epo) in invertebrate nervous systems
Erythropoietin
(Epo) is a
hematopoietic cytokine with multiple functions that are not exclusively
related
to vertebrate erythropoiesis. Epo is also expressed in the vertebrate
nervous
system where it serves important functions during neurodevelopment. Epo
has
been demonstrated to exert neuroprotective effects by interfering with
apoptotic pathways and to promote the regeneration of damaged neurites
in
mammalian nervous systems. There is accumulating evidence that the
functions of
Epo/Epo-receptor in nervous tissues are independent from effects on the
maturation of red
blood cells. Particularly, the existence of endogenous splice variants
and artificial derivatives of Epo that mediate neuroprotection but do
not stimulate erythropoiesis, suggested different Epo-responsive
receptors on erythroid progenitor cells and cells of other tissues
including the nervous system. While the classical Epo-receptor EpoR is present in erythrocyte progenitors and other tissues, non-hematopoietic tissues express additional types of Epo-receptors with different ligand profiles.
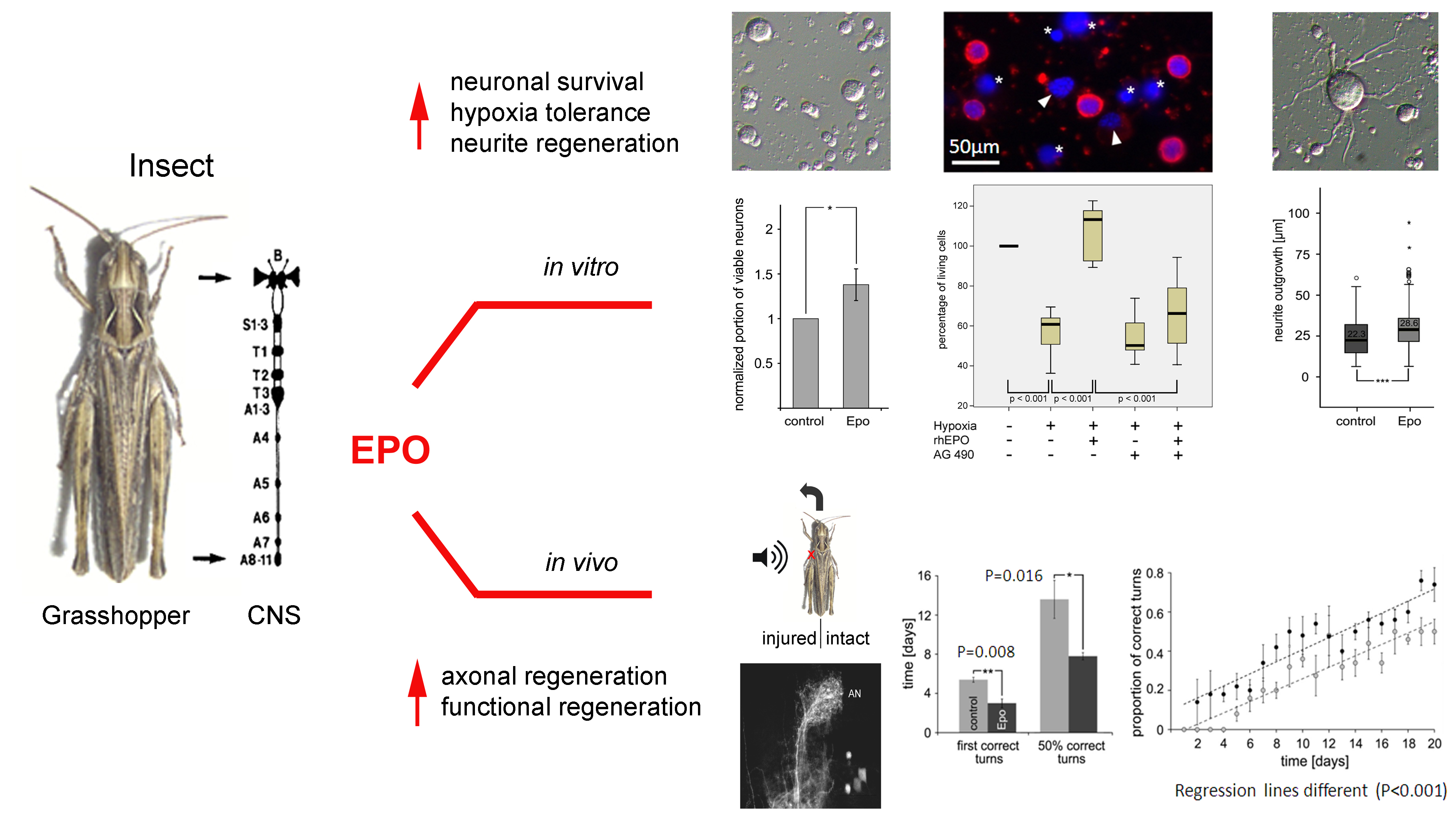
Insects and other invertebrates lack genes for Epo and EpoR. Nevertheless, similar to
its neuroprotective and neuroregenerative functions in mammals, recombinant
human Epo initiates beneficial mechanisms in grasshopper and beetle nervous
systems. In vitro, Epo increases
survival of primary cultured brain neurons in normoxic and hypoxic conditions
and promotes the regeneration of neurites. Epo interferes with apoptotic
mechanisms by activating AG490-sensitive Januskinase and STAT signaling. In vivo, Epo accelerates and improves
axonal regeneration and reestablishment of sound source localization after
crush injury of the tympanal nerve.
Locust and beetle brain neurons are also protected by the
non-erythropoietic human Epo splice variant EV-3 and other
non-erythropoietic Epo derivatives, suggesting that insect and
mammalian neuroprotective Epo receptors share common structures that
allow activation by the same non-erythropoietic agonists.
While EPO and its receptor
(EPOR) are only weakly expressed in normal adult brains, a variety of
stress factors including hypoxia can induce their enhanced expression
via accumulation of the transcription factor hypoxia-inducible factor-1
(HIF-1). EPO has been demonstrated to exert neuroprotective effects by
interfering with apoptotic pathways and to promote the regeneration of
damaged neurites in mammalian nervous systems. There is accumulating
evidence that the functions of EPO/EPOR in nervous tissues are
independent from effects on the maturation of red blood cells. The
neuroprotective and neurotrophic functions of EPO and EPOR in the
mammalian CNS may therefore be mediated by ancient evolutionary
conserved mechanisms whose characterisation could be facilitated by
studies on organisms without erythropoiesis, such as invertebrates.
Our studies suggest the presence of EPO/EPOR-like signalling pathways in grasshoppers and other invertebrates. In vivo,
human recombinant EPO accelerated the regeneration of auditory receptor
axons’ central projections after tympanal nerve crush, leading to
an earlier reestablishment of sound localisation in acoustically
communicating grasshoppers. In vitro, EPO increased the
survival of primary cultured insect brain neurons, promoted the
regeneration of neurites and increased cell survival in hypoxic
environments.
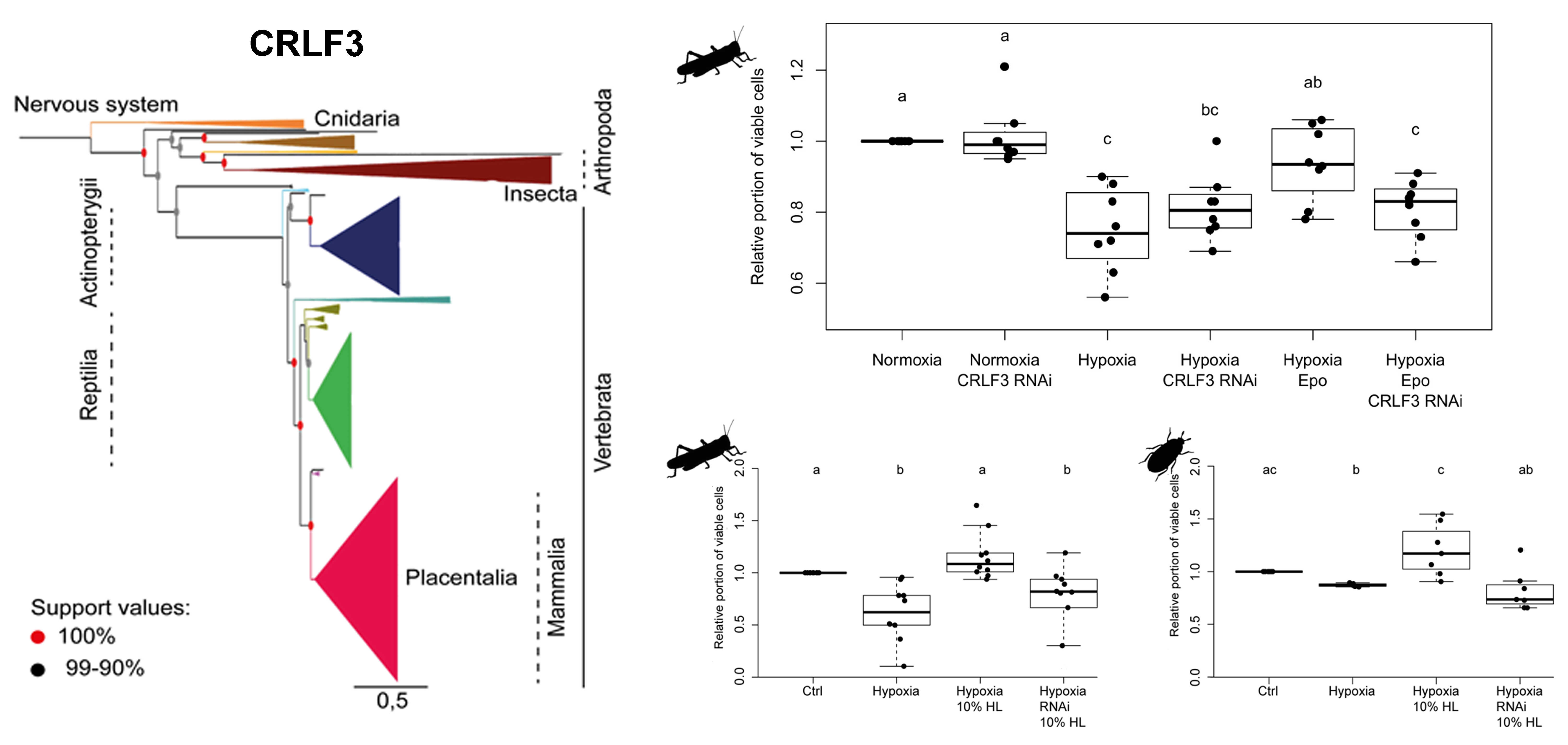
CRLF3
is highly conserved in eumetazoan species ranging from cnidarians to
mammals. RNAi-mediated knockdown of CRLF3 expression abolishes
Epo-mediated protection of hypoxia-challenged locust brain neuron in
vitro. Locust hemolymph extracts protect both locust and beetle neurons
against hypoxia-induced cell death.
Insect species like Locusta migratoria and Tribolium castaneum,
in which recombinant human Epo mediates neuroprotection, express a
single orthologue of the human orphan cytokine receptor-like factor 3
(CRLF3). CRLF3 belongs to the cytokine type 1 receptor family, which
also includes the hematopoietic cytokine receptors EpoR, thrombopoietin
receptor and GCSF receptor. Of this family only CRLF3 has orthologs in
invertebrates and it is highly conserved from cnidarians to mammals
including humans. Primary cultured insect neurons have been
demonstrated to lose
Epo-mediated protection in hypoxia following RNAi-mediated
downregulation of CRLF3, indicating that this receptor represents the
neuroprotective Epo-receptor in insects. Its
endogenous ligand (insects do not contain Epo) is contained in the
circulating hemolymph since locust hemolymph extract protects both
locust and beetle neurons from hypoxia-induced apoptosis through a
CRLF3-dependentmechanism.
Pro-apoptotic functions of insect acetylcholinesterase
Apoptosis
contributes to the development of structured organs, enables the renewal of
adult tissues, mediates the removal of compromised or malfunctioning cells and
is critically involved in various degenerative diseases. Recent studies
indicated that an early ancestor of all metazoans already possessed most
components of the complex vertebrate-typical apoptotosis regulatory network.
Consequently, the well investigated comparatively simple apoptotic networks of
the model organisms C. elegans and D. melanogaster resulted from a
secondary reduction of ancient complexity.
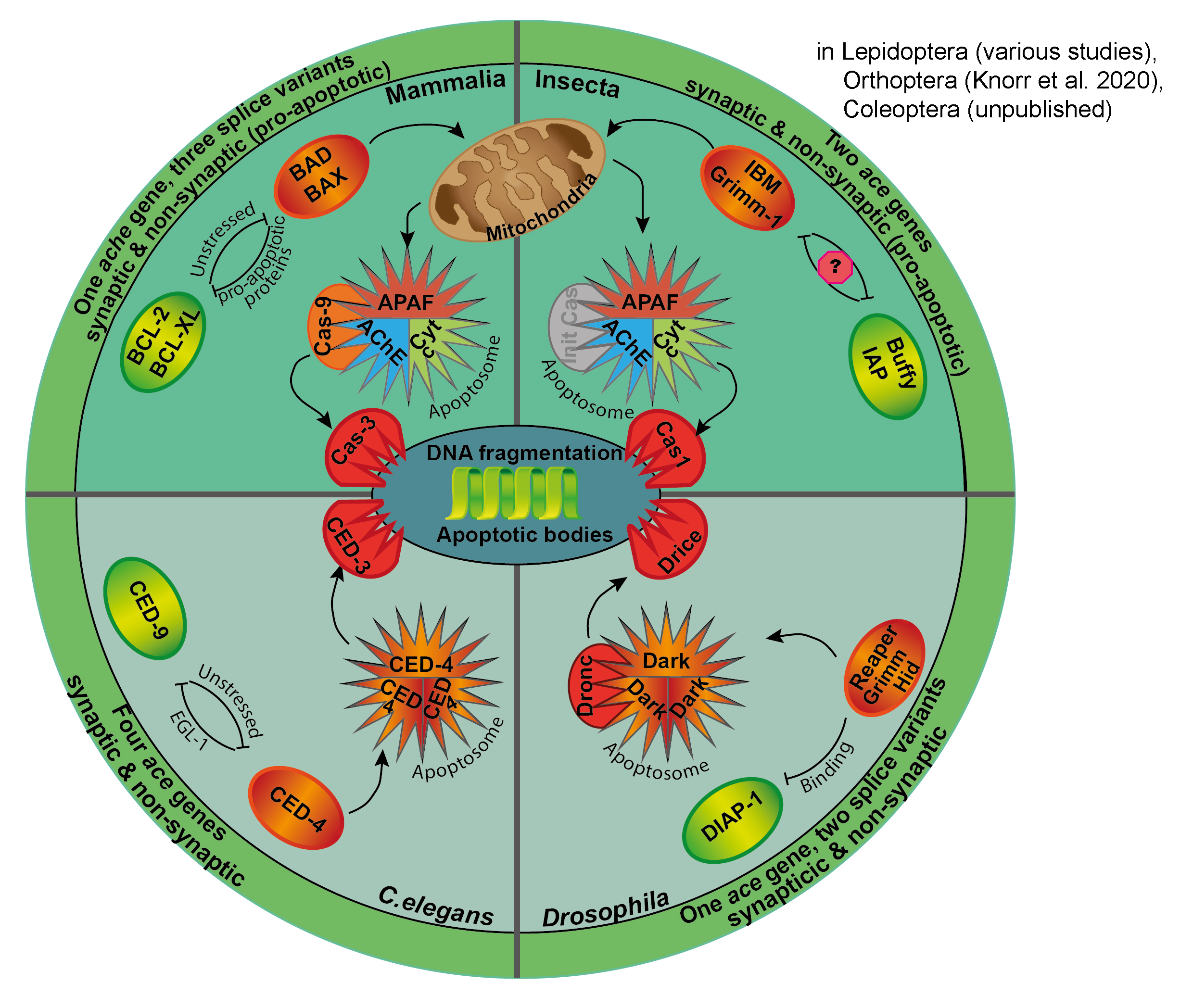
Pizza of Death
Summary of apoptotic pathways in mammals, nematodes (C. elegans) and insects (D. melanogaster, lepidoptera (S. frugiperda and B. mori) and orthoptera (L. migratoria)). Experimental data from lepidopteran, coleopteran and orthopteran
insects indicate the contribution of cytochrome c and
acetylcholinesterase to insect apoptosis. Detailed description in Knorr et al. 2020).
We study apoptotic mechanisms in L.
migratoria and T. castaneum,
to
(1) determine stimuli that promote apoptosis, (2) characterize the
molecular
mechanisms underlying apoptosis and (3) identify protective pathways
that
interfere with apoptotic cell death. Along these lines, we demonstrated
that acetylcholinesterase activity promotes apoptosis of primary insect
neurons. Hence, acetylcholinesterase may have a similar role in
apoptosome formation as it has previously been described for various
mammalian cells types.
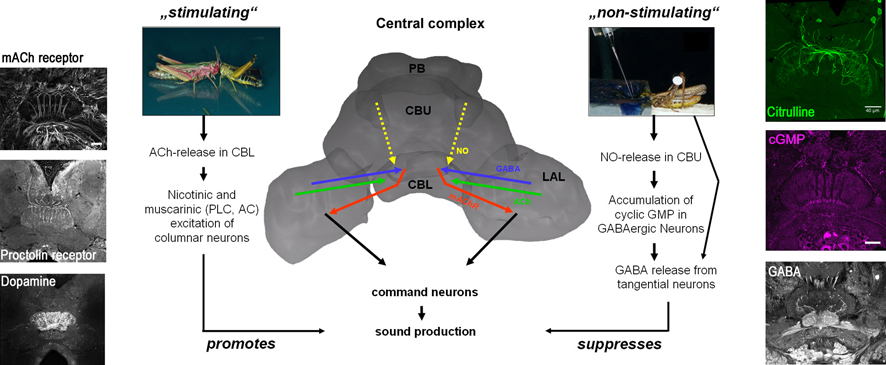
Sound production
depends on the balance of fast and slow excitation and inhibition in
central complex neuropils and various transmitters, modulators and
intracellular signalling pathways that promote (ACh, proctolin,
dopamine) or suppress (GABA, NO) sound production have been identified
by pharmacological stimulation and confirmed by anatomical studies. Two
of these signaling pathways have been associated with particular
behavioral situations. Hearing and recognizing conspecific song
activates cholinergic projections to the central complex leading to
both nicotinic excitation of yet unknown targets and muscarinic
excitation of columnar neurons. The latter is mediated by phospholipase
C and adenylyl cyclase-initiated intracellular signaling pathways.
Expression of muscarinic ACh receptors in the central complex is
limited to a subset of columnar neurons with their cell bodies located
in the pars intercerebralis, which are thought to contact pre-motor
elements in the lateral accessory lobes. In contrast, a different set
of pars intercerebralis neurons with columnar projections in the upper
division of the central body and tangential neurons with cell bodies in
the ventro-median protocerebrum contain the enzyme nitric oxide
synthase and accumulate citrulline in situations that are unfavorable
for sound production. Since liberation of nitric oxide in the central
body inhibits sound production via soluble guanylyl cyclase activation
and cyclic GMP production in the central body lower division, these
citrulline-accumulating central complex neurons may translate
inappropriate behavioral situations into nitric oxide-mediated
suppression of sound production. By applying multiple antibodies
directed against components of signaling pathways that contribute to
the control of grasshopper sound production to the central complex and
conducting physiological studies on pre-identified central complex
neurons in primary cell culture, we are attempting to identify the
points of convergence of different signals in order to trace the flow
of information within the central complex.
Back to top
Control of grasshopper sound production by the central complex
The central complex in the
protocerebrum coordinates the type, intensity and timing of sound signals used
for mate attraction, courtship and rivalry of acoustically communicating
grasshoppers.
Sound production depends on
the balance of fast and slow excitation and inhibition in central complex
neuropils and various transmitters, modulators and intracellular signalling
pathways that promote (ACh, proctolin, dopamine) or suppress (GABA, NO) sound
production have been identified by pharmacological stimulation and confirmed by
anatomical studies. Two of these signaling pathways have been associated with
particular behavioral situations. Hearing and recognizing conspecific song
activates cholinergic projections to the central complex leading to both
nicotinic excitation of yet unknown targets and muscarinic excitation of
columnar neurons. The latter is mediated by phospholipase C and adenylyl
cyclase-initiated intracellular signaling pathways. Expression of muscarinic
ACh receptors in the central complex is limited to a subset of columnar neurons
with their cell bodies located in the pars intercerebralis, which are thought
to contact pre-motor elements in the lateral accessory lobes. In contrast, a
different set of pars intercerebralis neurons with columnar projections in the
upper division of the central body and tangential neurons with cell bodies in
the ventro-median protocerebrum contain the enzyme nitric oxide synthase and
accumulate citrulline in situations that are unfavorable for sound production.
Since liberation of nitric oxide in the central body inhibits sound production
via soluble guanylyl cyclase activation and cyclic GMP production in the
central body lower division, these citrulline-accumulating central complex
neurons may translate inappropriate behavioral situations into nitric
oxide-mediated suppression of sound production. By applying multiple antibodies
directed against components of signaling pathways that contribute to the
control of grasshopper sound production to the central complex and conducting
physiological studies on pre-identified central complex neurons in primary cell
culture, we are attempting to identify the points of convergence of different
signals in order to trace the flow of information within the central complex.
Sound production
depends on the balance of fast and slow excitation and inhibition in
central complex neuropils and various transmitters, modulators and
intracellular signalling pathways that promote (ACh, proctolin,
dopamine) or suppress (GABA, NO) sound production have been identified
by pharmacological stimulation and confirmed by anatomical studies. Two
of these signaling pathways have been associated with particular
behavioral situations. Hearing and recognizing conspecific song
activates cholinergic projections to the central complex leading to
both nicotinic excitation of yet unknown targets and muscarinic
excitation of columnar neurons. The latter is mediated by phospholipase
C and adenylyl cyclase-initiated intracellular signaling pathways.
Expression of muscarinic ACh receptors in the central complex is
limited to a subset of columnar neurons with their cell bodies located
in the pars intercerebralis, which are thought to contact pre-motor
elements in the lateral accessory lobes. In contrast, a different set
of pars intercerebralis neurons with columnar projections in the upper
division of the central body and tangential neurons with cell bodies in
the ventro-median protocerebrum contain the enzyme nitric oxide
synthase and accumulate citrulline in situations that are unfavorable
for sound production. Since liberation of nitric oxide in the central
body inhibits sound production via soluble guanylyl cyclase activation
and cyclic GMP production in the central body lower division, these
citrulline-accumulating central complex neurons may translate
inappropriate behavioral situations into nitric oxide-mediated
suppression of sound production. By applying multiple antibodies
directed against components of signaling pathways that contribute to
the control of grasshopper sound production to the central complex and
conducting physiological studies on pre-identified central complex
neurons in primary cell culture, we are attempting to identify the
points of convergence of different signals in order to trace the flow
of information within the central complex.
Back to top
Modulation of female grasshoppers’ reproductive behaviour by nitric oxide and juvenile hormone
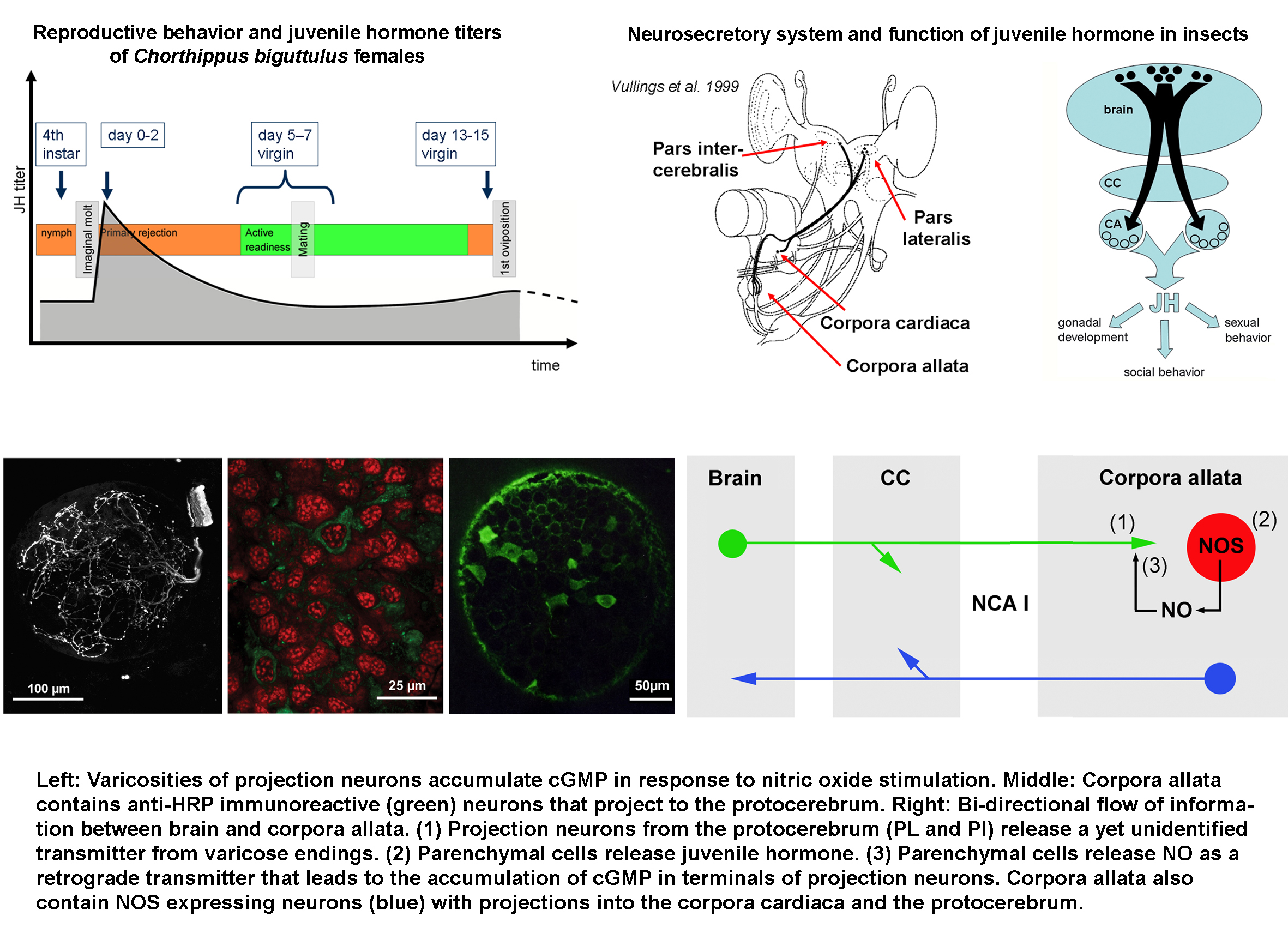
The sexual behaviour of
female Ch. biguttulus grasshoppers changes with age, oocyte cycle and
mating experience (for a description see Wirmer et al. 2010). Immediately after their imaginal molt, females
reject all male mating attempts (“primary rejection”). Within a few days,
females enter the state of “active copulatory readiness”, a state of high
receptivity recognized by singing in response to male stridulation, orientation
and active walking towards the partner. After mating, females assume the state
of “secondary rejection” which lasts for several days and may end with another
period of “copulatory readiness”.
Previous studies on various grasshopper species implicated two signalling systems
in the control of female reproductive states, nitric oxide/cGMP signalling in
the brain and juvenile hormone (JH) released from the corpora allata. Ch.
biguttulus females that are injected with the nitric oxide synthase
inhibitor aminoguanidine respond longer and more frequently to male calling
songs (Weinrich et al. 2008) while grasshopper females that lack JH remain in
a rejective state and do not stridulate (Loher 1962).
We evaluate the effects of NO and juvenile hormone JH on reproduction related
behaviors of female grasshoppers and particularly look for interactions in the
brain and/or the corpora allata or for simultaneous regulation of both
substances by upstream systems. We assess female responsiveness to male calling
songs after diverse treatments that interfere with JH titers or NO production.
Cellular sources and targets of NO as well as the distribution of neuroactive
signals thought to control JH synthesis are investigated by
immunocytochemistry. In contrast to previous beliefs that the corpora allata
are regulated by the brain, we demonstrated the presence of neurons that
project from the corpora allata to the protocerebrum, suggesting a more complex
bi-directional information flow between brain and neurosecretory organs.
Immediately after
their imaginal molt, females perform “primary rejection”
against male mating attempts. Within a few days, females enter the
state of “active copulatory readiness”, a state of high
receptivity recognized by singing in response to male stridulation,
orientation and active walking towards the partner. After mating
females assume the state of “secondary rejection” which
lasts for several days and may end with the next period of
“copulatory readiness”.
Previous studies on various grasshopper species implicated two
signalling systems with the control of female reproductive states,
nitric oxide/cGMP signalling in the brain (Weinrich et al. 2008) and juvenile hormone (JH) released from the corpora allata (Loher 1962). Ch. biguttulus
females that are injected with the nitric oxide synthase inhibitor
aminoguanidine respond longer and more frequently to male calling songs
(Weinrich et al. 2008) while grasshopper females that lack JH remain in a rejective state and do not stridulate (Loher 1962).
We evaluate the effects of nitric oxide (NO) and juvenile hormone (JH)
on reproduction related behaviour of female nightingale grasshoppers
and particularly look for interactions in the brain or the corpora
allata or for simultaneous regulation of both substances by upstream
sytems. We assess female responsiveness to male calling songs after
diverse treatments that interfere with JH titers or NO production. JH
titers in the hemolymph of females in different reproductive states and
after different pharmacological treatments are determined in
cooperation with the University of Central Florida and the University
of Bayreuth. Cellular sources and targets of NO as well as the
distribution of neuroactive signals thought to control JH synthesis are
investigated by immunocytochemistry.
Back to top
Acoustic communication and social behavior of Drosophila melanogaster
Drosophila
melanogaster offers an arsenal of molecular genetic tools to identify the functions of
individual genes and proteins, their interaction partners within
cellular/molecular pathways and their impact on physiology and behavioral
performance. Making use of this large variety of genetic methods to manipulate
the formation and function of its nervous system, studies on Drosophila have increased our knowledge
about nervous development, plasticity including learning and memory formation
and the control of sexually dimorphic complex interactive behaviours like
courtship and aggression.
Human
neuro-developmental disorders such as autism spectrum disorders, schizophrenia,
attention deficit hyperactivity disorders and Tourette syndrome are believed to
result from interplay of multiple genetic risk factors with environmental
stimuli. In many cases defects in synaptogenesis, synaptic maintenance and
plasticity account for phenotypes that include deficits in social behavior,
communication and cognitive functions. Drosophila’s
behaviour, including its well-decribed social behavior, is increasingly used to
study mechanisms underlying heritable human neuro-developmental disorders,
pinpointing the contribution of genetic risk factors for these conditions. As
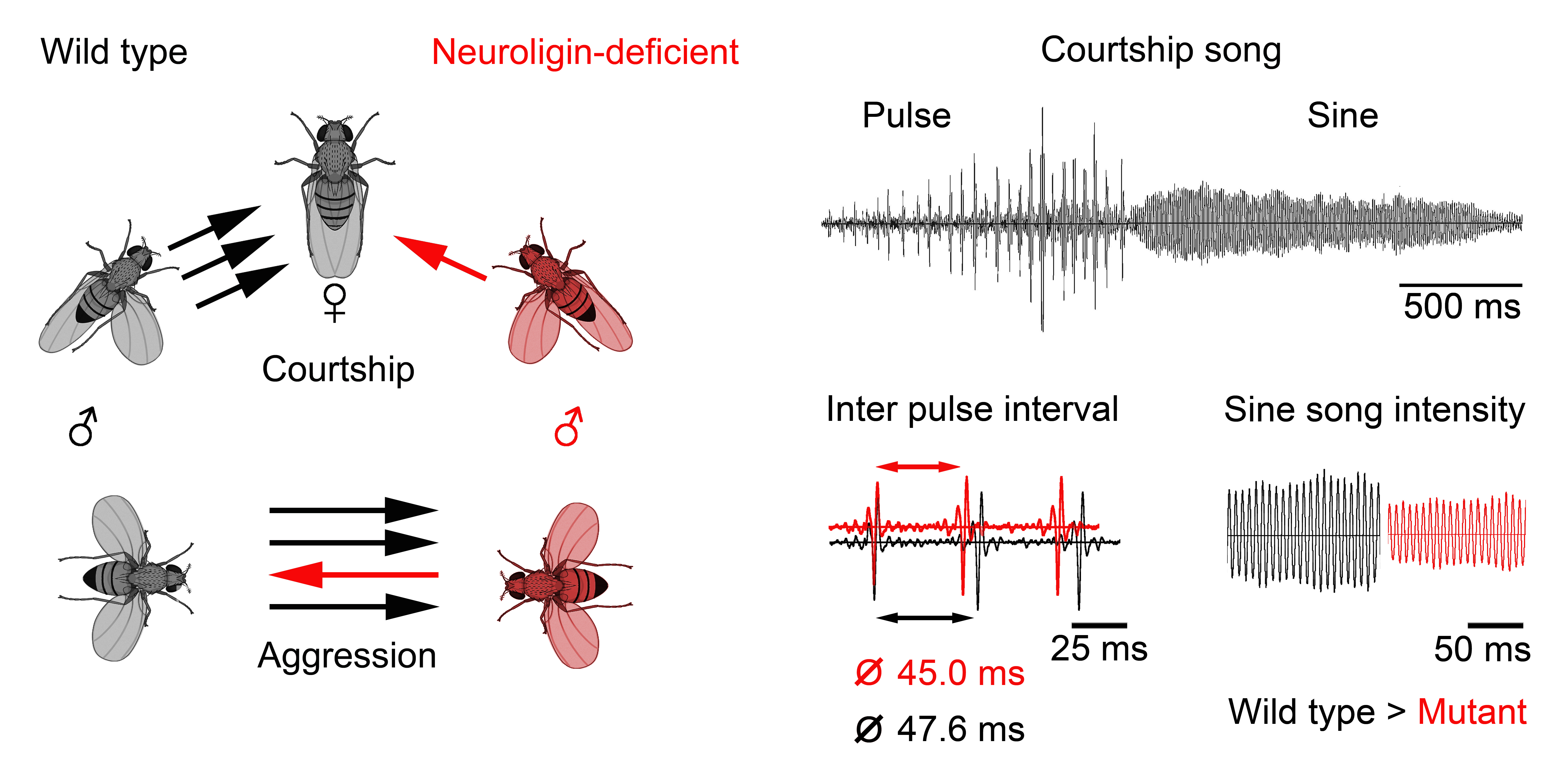
one example, we expose neuroligin 2-deficient Drosophila to behavioural tests addressing their social
interactions, space-dispersal, and behavioural switching and analysed their
acoustic communication patterns. Neuroligins are a family of phylogenetically
conserved postsynaptic adhesion molecules that code for postsynaptic cell
adhesion molecules whose intracellular domains bind to synaptic scaffolding
proteins while extracellular domains assemble with presynaptic Neurexins.
Mutations in neuroligin genes have
been identified as risk factors for the development of autism spectrum
disorders (ASDs). We show that deletion of the dnl2 gene, coding for one of four Neuroligin isoforms, alters
acoustic communication signals, affects the transition between different
behaviours and impairs social interactions in Drosophila melanogaster. dnl2-deficient
flies maintain larger distances to conspecifics and males perform less
female-directed courtship and male-directed aggressive behaviours while the
patterns of these behaviours and general locomotor activity resembled those of
wild type controls. Since olfactory, visual and auditory perception were not
altered in dnl2-deficient mutants,
reduced social interactions seem to result from altered excitability in central
nervous neuropils that initiate social behaviours. Our results demonstrate that
neuroligins are phylogenetically conserved not only regarding their structure
and direct function at the synapse but also their fine-tuning of synaptic
function in brain circuits that regulate social behaviours dates back to common
ancestors of humans and flies.
As two examples of intraspecific interactions, courtship and agonistic behaviour were described in Drosophila
almost a century ago (Sturtevant 1915) and a large amount of literature
concerning different forms of courtship behaviour in various subgroups
of Drosophila is available. Male Drosophila acquire and
defend territories in order to attract females for reproduction. Both,
male-directed agonistic behaviour and female-directed courtship consist
of series of recurrent stereotyped components. Various studies
demonstrated the importance of species-specific sound patterns
generated by wing vibration as being critical for male courtship
success. We have started to analyse the patterns and importance of
sound signals generated during agonistic interactions of male Drosophila melanogaster.
In contrast to acoustic courtship signals that consist of sine and
pulse patterns and are generated by one extended wing, agonistic
signals lack sine-like components and are generally produced by
simultaneous movements of both wings. Though intra-pulse oscillation
frequencies (carrier frequency) are identical, inter-pulse intervals
are twice as long and more variable in aggression signals than in
courtship songs, where their precise temporal pattern serves species
recognition. Acoustic signals accompany male agonistic interactions
over their entire course but occur especially frequently after tapping
behaviour which serves to identify the gender of the interaction
partner. Since similar wing movements may either be silent or generate
sound and wing movements with sound have a greater impact on the
receiver’s subsequent behaviour, sound seems to be generated
intentionally to serve as acoustic signal during fruitfly agonistic
encounters.
Evolution and function of stick insects' "mushroom sensilla"
Some ground-dwelling stick insects contain sensilla of mushroom-like
shapes (“mushroom-sensilla”) of yet unknown function.
Depending on the species,
these sensilla are either arranged in two lateral fields on the
probasissternit, or in one field on the profurcasternit, or in all
three locations (Rehn & Rehn 1938).
They consist of a cone-shaped shaft, about 50-75µm long, with a
grooved surface, ending in a mushroom-shaped enlargement at the apex
and a torus-shaped socket. „Mushroom-sensilla“ contain no
pores, suggesting that they don’t function as chemoreceptors.
Ultrastructural analysis revealed that each sensillum is innervated by
only one sensory cell. Central projections of these sensory cells
terminate in a large median area of the prothoracic ganglion and a
smaller, caudally extending lateral branch. All central projections
terminate in the ventral association center (vVAC) or the lateral VAC
(lVAC), suggesting that the „mushroom-sensilla“ could
represent a type of mechanoreceptor (Johnson & Murphey 1985).
„Mushroom-sensilla“ appear in several ground-dwelling
species which are not closely related to each other which leads to the
conclusion that the appearance is due to a convergent development as an
adaption to a ground-dwelling way of life. A more parsimonious
explanation for the appearance of these sensilla would be that they
were already present in a ground-dwelling ancestor of the stick
insects. Subsequently, they were not expressed for several generations,
because stick insects evolved a canopy-dwelling way of life, for which
„mushroom-sensilla“ provided no particular advantage. But
with some species returning to the ground
„mushroom-sensilla“ re-evolved. Several other studies, e.g.
on the development of a molar teeth in the lynx (Lynx lynx) (Werdelin 1987) or on the development of wings in stick insects (Whiting et al. 2003),
revealed that re-evolution is a possible mechanism. If the
„mushroom-sensilla“ re-evolved, their complex structures
and their functions in different species should be very similar, making
a convergent evolution unlikely.
more