The transcriptional antiterminator GlcT as an
example for a regulated protein-RNA interaction
 Protein-RNA-interactions
are involved in basic biological processes such as intron
splicing, enzymatic catalysis, protein secretion, and regulation of gene
expression. In many instances, the sequence specific binding of a protein to
its RNA target is modulated by signals. While the importance of protein-RNA
interactions is well recognized, the knowledge of its molecular details has
remained limited. We want to understand protein-RNA recognition and its
control by external signals in dynamic and structural terms. To this end, we
characterize the structural basis of sequence-specific RNA binding of the
transcriptional antiterminator GlcT
from Bacillus subtilis and its control. Since we
use genetic methods in addition to biochemical, cristallographic
and NMR methods, a bacterial model is most suited. We chose the regulation of
glucose permease of the phosphotransferase
system from B. subtilis since this protein is both
a glucose receptor and a signal-dependent kinase
for different substrates. The signals generated by this protein regulate
important cellular processes such as horizontal gene transfer, chemotaxis and carbon catabolite
repression. Expression of this signalling protein is induced in the presence
of glucose by the sequence specific RNA-binding protein GlcT.
The general importance of signalling chains made up of a receptor, a protein kinase and an RNA binding protein adds relevance to our
research project. We are going to test our hypothesis of a reversible phosphorylation of GlcT by the
glucose receptor by an in vitro approach. The reconnaissance between GlcT and its RNA targets is to be understood in its
molecular details using in vitro binding assays and an analysis of suppressor
mutations in the RNA-binding domain of GlcT that
allow binding to altered RNA sequences. Figures: The PTS Antitermination
at the ptsGHI operon
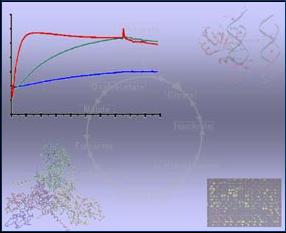
Demonstration of protein-RNA interaction by BIAcore
analysis Model of GlcT phosphorylation Protein-RNA-interactions
are involved in basic biological processes such as intron
splicing, enzymatic catalysis, protein secretion, and regulation of gene
expression. In many instances, the sequence specific binding of a protein to
its RNA target is modulated by signals. While the importance of protein-RNA
interactions is well recognized, the knowledge of its molecular details has
remained limited. We want to understand protein-RNA recognition and its
control by external signals in dynamic and structural terms. To this end, we
characterize the structural basis of sequence-specific RNA binding of the
transcriptional antiterminator GlcT
from Bacillus subtilis and its control. Since we
use genetic methods in addition to biochemical, cristallographic
and NMR methods, a bacterial model is most suited. We chose the regulation of
glucose permease of the phosphotransferase
system from B. subtilis since this protein is both
a glucose receptor and a signal-dependent kinase
for different substrates. The signals generated by this protein regulate
important cellular processes such as horizontal gene transfer, chemotaxis and carbon catabolite
repression. Expression of this signalling protein is induced in the presence
of glucose by the sequence specific RNA-binding protein GlcT.
The general importance of signalling chains made up of a receptor, a protein kinase and an RNA binding protein adds relevance to our
research project. We are going to test our hypothesis of a reversible phosphorylation of GlcT by the
glucose receptor by an in vitro approach. The reconnaissance between GlcT and its RNA targets is to be understood in its
molecular details using in vitro binding assays and an analysis of suppressor
mutations in the RNA-binding domain of GlcT that
allow binding to altered RNA sequences. Figures: The PTS Antitermination
at the ptsGHI operon
Demonstration of protein-RNA interaction by BIAcore
analysis Model of GlcT phosphorylation
Key references
Langbein, I., Bachem, S. & Stülke, J.
(1999) Specific interaction of the RNA binding
domain of the Bacillus subtilis
transcriptional antiterminator GlcT with its RNA target, RAT. J. Mol. Biol.
293: 795-805.
Ludwig, H., Homuth, G., Schmalisch,
M., Dyka, F. M., Hecker,
M. & Stülke, J. (2001) Transcription of glycolytic genes and operons in
Bacillus subtilis:
Evidence for the presence of multiple levels of control of the gapA operon. Mol. Microbiol. 41:
409-422.
Stülke, J. (2002)
Control of transcription termination in bacteria by RNA-binding proteins by
RNA-binding proteins that modulate RNA structures. Arch. Microbiol.
177: 433-440.
Meinken, C., Blencke, H.-M., Ludwig, H. & Stülke,
J. (2003) Expression of the glycolytic gapA operon in Bacillus subtilis: differential syntheses of proteins encoded
by the operon. Microbiology 149: 751-761.
Schmalisch, M., Bachem, S. & Stülke, J. (2003)
Control of the Bacillus subtilis antiterminator
protein GlcT by phosphorylation:
Elucidation of the phosphorylation chain leading to
inactivation of GlcT. J. Biol. Chem. 278:
51108-51115.
Schilling, O., Langbein, I., Müller,
M., Schmalisch, M. & Stülke,
J. (2004) A protein-dependent riboswitch controlling
ptsGHI operon expression in Bacillus
subtilis: RNA structure rather than sequence
provides interaction specificity. Nucl. Acids Res. 32:
2853-2864.
|

