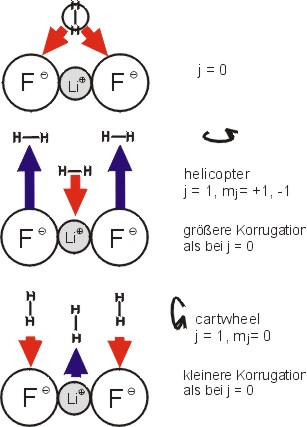
The cartoon illustrates how the attractive (arrows pointing down) and repulsive (arrows pointing up) electrostatic forces between the ions on a LiF surface and a hydrogen molecules lead to a situation in which the molecules in different rotational states experience different apparent corrugations of the surface.