|
Department of
General Microbiology Research group Dr. Boris Görke Regulation
of carbohydrate metabolism in the model bacteria Escherichia coli and Bacillus
subtilis |
||
|
Since its
discovery 150 years ago, the Gram-negative bacterium Escherichia coli has certainly become the best understood living
organism. It not only serves as a model organism in basic research but also as
a “workhorse” in almost all fields of modern molecular biology. Moreover, E. coli and some of its closest
relatives are important pathogens causing numerous infection diseases. Similarly,
Bacillus subtilis, a non-pathogenic
spore-forming soil bacterium, has become a useful paradigm for most of the
Gram-positive bacterial world. The attraction of sporulation as a model for a
simple developmental process and the ease of genetic manipulation made B. subtilis a preferred subject for
detailed investigation. |
||
|
Our research
focuses on the signal transduction pathways and regulatory mechanisms controlling
carbohydrate metabolism in these bacteria. The flow rate of carbon through
the central metabolic pathways provides the primary signal to which all other
cellular processes must be adapted and vice versa. Hence, bacteria evolved
highly integrated regulatory circuits that coordinate carbohydrate
utilization with virtually all other cellular processes. It is interesting to
see, how two evolutionary distant bacterial species evolved different
solutions for one and the same problem. |
||
|
I. Scientific background and Research projects |
||
|
A. Control of carbohydrate metabolism by reversible
protein phosphorylation |
||
|
In bacteria, transport
of many carbohydrates across the cytoplasmic membrane is achieved by the phosphotransferase
system (PTS). Transport of a substrate is coupled to its phosphorylation and
involves a phosphorylation cascade composed of two general protein kinases EI
and HPr, and of the sugar specific transporters (EIIs). |
||
|
In addition,
the PTS represents a highly integrated signal transduction system coupling
enzyme activities and expression of hundreds of genes to the nature of available
carbon sources. The signal that initiates the various signal transduction
chains is often provided by the phosphorylation state of the PTS proteins,
which indirectly reflects transport activity. In E. coli the IIAGlc domain of the glucose transporter serves as the processing unit
responsible for the well-known regulatory phenomena called “inducer
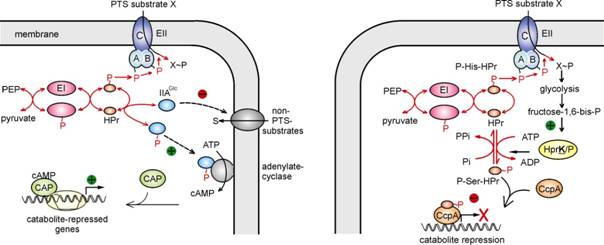
exclusion” and “carbon catabolite repression” (CCR), respectively (Fig. 1
left). In B. subtilis a quite
different mechanism exists (Fig. 1, right). In this bacterium, HPr can be
phosphorylated by the HPr-kinase HPrK/P at a second site, Ser-46.
Phospho-Ser46-HPr acts as co-repressor for the carbon catabolite control
protein CcpA. The complex of P~Ser46-HPr/CcpA binds to specific sites in the
DNA and prevents expression of many catabolic genes. This process is
triggered by central glycolytic metabolites like fructose-1,6-bis-phosphate which
is required for activation of HPrK (Fig. 1, right). In an ongoing research
topic, we are investigating the detailed molecular mechanisms governing CCR
in B. subtilis. Our analyses
suggest that further factor(s) exist that contribute to CCR in
this organism.
Fig. 1. Mechanisms of carbon catabolite repression in
E. coli (left) and B. subtilis (right). In addition,
proteins of the PTS regulate a variety of physiological processes, e.g.
chemotaxis towards PTS sugars and carbon storage. Moreover, the activities of
transcriptional regulatory proteins that contain so-called PTS regulation
domains (PRDs) are controlled by the PTS. These regulators in turn control
genes coding for functions involved in PTS sugar utilization. In another ongoing
project, we focus on the putative regulatory role(s) of paralogs of the central
phosphotransferases HPr, EI and IIAGlc. Both, B.
subtilis as well as E. coli,
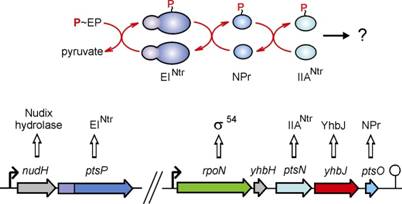
encode those proteins but their functions are less understood. In E. coli, those paralogs are encoded in
the rpoN operon (Fig. 2). The corresponding proteins EINtr,
NPr and IIANtr, were shown to form a phosphorylgroup transfer
chain that works in parallel to the canonical PTS (Fig. 2). However, a final
phosphoryl group acceptor is unknown making it unlikely that this system has
a transport function. In contrast, our analyses suggest that this system
plays a
role in regulation of uptake of K+ rather than of a carbohydrate.
Fig. 2. PTS homologs encoded in the E. coli rpoN operon. B.
Regulation of carbohydrate metabolism by small non-coding RNAs. In the past decade small RNAs (sRNAs) turned out to
be a novel class of regulators of gene expression in all kingdoms of life. In
E. coli ~100 of such sRNAs have been identified. However,
their physiological functions are known in just a few cases and the molecular
mechanisms underlying gene regulation are not well understood. At present, trans-encoded base-pairing sRNAs are
known to play regulatory roles in the response to iron starvation, to cell
envelope stress and to other stress conditions. Evidence accumulates that
regulation of carbohydrate metabolism is also a major domain of sRNA
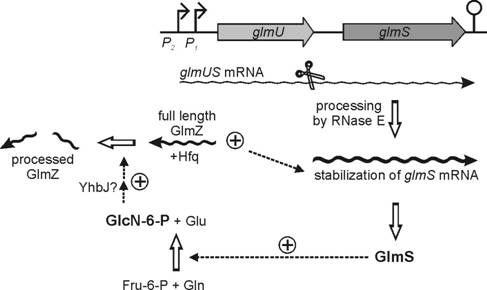
regulation in E. coli. We recently discovered that in E. coli expression of the central metabolic enzyme
glucosamine-6-phosphate synthase (GlmS) is feedback-regulated by its product
glucosamine-6-phosphate (GlcN-6-P) in a process that depends on the sRNA GlmZ
(see model in Fig. 3). GlmS initiates the hexosamine pathway leading to the
formation of precursor molecules for the biosynthesis of peptidoglycan of
bacterial cell walls. The sRNA GlmZ is subject to processing and we found
that the full-length form of GlmZ activates glmS expression. When the intracellular GlcN-6-P concentration
decreases, full-length GlmZ accumulates and in turn stabilizes the mono-cistronic
glmS mRNA that derives from
processing of the glmUS
co-transcript. In addition, we identified the protein YhbJ, which is encoded
in the rpoN operon (Fig. 2), as a
regulator of GlmZ processing. Furthermore, a second sRNA, GlmY, also affects
expression of glmS. Our major goals
are to clarify the interplay of the two sRNAs in the control of glmS, to identify the GlcN-6-P sensory
molecule in the system, and to reveal the molecular mechanism by which YhbJ
controls processing of the sRNAs.
Fig. 3. Model for feedback regulation of glmS expression by GlcN-6-P (adapted from:
Kalamorz et al., 2007). II. Key
references and recommended reading Kalamorz, F., Reichenbach, B., März,
W., Rak, B. and B. Görke (2007) Görke, B. and J. Deutscher (2007) The regulatory
functions of histidyl-phosphorylated HPr in bacilli. In: Global
regulatory networks in Bacillus subtilis, edited by Y. Fujita, Transworld
Research Network, Trivandrum, India; pp. 1-37; ISBN: 978-817895-299-4. Singh, K. D., Halbedel, S., Görke, B. and J. Stülke (2007) Control of
the phosphorylation state of the HPr protein of the phosphotransferase system
in Bacillus subtilis: Implication
of the protein phosphatase PrpC. J.
Mol. Microbiol. Biotechnol., 13,
165-171. Reichenbach, B.,
Breustedt, D.A., Stülke, J., Rak, B. and B. Görke (2007) |
||
|
|
||
|
|
|
|