
Francisco Welter-Schultes
II. Zoologisches Institut der Universität
Berliner Str. 28
D-37073
Göttingen, Germany
E-mail:
![]()
.
Homepage
Human-dispersed land snails in Crete, with special reference to Albinaria (Gastropoda: Clausiliidae).
This paper was published in Biologia Gallo-Hellenica. I am the author of
the paper and have no reprints any more. Because this paper is frequently requested,
I decided to create this internet file.
Biologia Gallo-Hellenica is a Greek journal dedicated to the biology
of Greece and adjacent regions. If you have any questions about the journal
or would like to subscribe the journal, please write to the editor
Prof. Dr. J. Matsakis
Université P. Sabatier
Neurobiologie - Entomologie
118, Rue de Narbonne
F-31062 Toulouse, France
Last modified 2.10.2002.
|
Francisco Welter-Schultes |
E-mail:
|
.
WELTER-SCHULTES, F. W. (1998):
Human-dispersed land snails in Crete, with special reference to
Albinaria (Gastropoda: Clausiliidae). -- |
.
This is the text of this paper. Figures are below.
.
HUMAN-DISPERSED LAND SNAILS IN CRETE, WITH SPECIAL REFERENCE TO ALBINARIA (GASTROPODA: CLAUSILIIDAE)
F. W. WELTER-SCHULTES
Institut für Zoologie und Anthropologie der Universität, Berliner Str. 28, D-37073 Göttingen, Germany
Abstract
Land snails were frequently transported by humans to Aegean islands. At least
16 snail species were introduced to Crete from different Mediterranean regions.
Most of them reached Crete in the antiquity. Besides of the edible Helix the
introduced snails were dispersed accidentally.
The more than 20 Cretan Albinaria species were not introduced but are local
endemics. Their distribution patterns are difficult to understand. Some Albinaria
populations in Crete originated through accidental dispersal by humans. Here
I suggest a method to recognize human-dispersed Albinaria populations in the
field. The results indicate that Albinaria was transported together with stones
used for construction or other purposes. I also present geographic positions
where such populations were found and hypotheses on their history of dispersal.
Based on the analysis of two particular human-dispersed populations (at Knosós
and on the island of Día) the average dispersal velocity of Albinaria
is calculated. Under optimal dispersal conditions it may range between 2.0 and
2.5 m yr-1, but facing a competing species it is reduced to less than 0.6 m
yr-1. Human-dispersed Albinaria populations in Crete are relatively rare and
do not occupy large ranges. Most populations are natural and their phylogeography
has to be explained otherwise.
Key words: Land snails, Albinaria, dispersal, historical ecology, human impact, introduced species, invasions, island biogeography, phylogeography, Crete, Greece.
Introduction
Many land snails are considered to be introduced to Crete and the south Aegean
islands from other Mediterranean regions, such as northern Africa (Rumina decollata,
Helix nucula, Cochlicella acuta, Theba pisana), the Iberian peninsula (Microxeromagna
armillata, Xerotricha conspurcata, Caracollina lenticula), Italy or Sicily (Xeromunda
candiota, Trochoidea pyramidata, Helix aspersa, Helix aperta), the Black Sea
region (Xeropicta krynickii), Turkey or Syria (Oxychilus camelinus, Oxychilus
cyprius). Deroceras panormitanum was probably introduced from the Greek mainland
to Andikíthira (WIKTOR et al. 1994). Also within the Aegean region, snails
were transported from one island to another. Deroceras oertzeni, a local endemic
of the Cyclades, was introduced to eastern Crete (WIKTOR et al. 1994). Albinaria
cretensis was transported from northwestern Crete to Lésvos (NORDSIECK
1977, BANK 1988). Tandonia totevi, originally living in the southern Aegean,
was introduced to Mílos, Kímolos, Greek Macedonia and to the Rodópi
Mountains, Bulgaria (WIKTOR et al. 1994). Anthropochorous dispersal of snails
continues to take place in Crete. Living Helix aspersa, Eobania vermiculata,
Theba pisana and Xerocrassa cretica are commonly sold on the Cretan markets.
Apart from some general remarks (FALKNER 1990), human-based dispersal is rarely
mentioned in studies of the eastern Mediterranean land snail fauna. In MYLONAS
(1982, 1984), the influence of humans on the snail fauna of the Kikládes
is briefly discussed. In an attempt to shed more light on the gap between biological
sciences and human history, human-based dispersal by crusaders was suggested
by GLAUBRECHT (1993) for the widespread eastern Mediterranean Levantina spiriplana.
However, this species does not live in Crete.
Snails introduced to Crete and surrounding islands
Next to the insects, land snails belong to the most abundant animals on the
Greek islands. The following 16 species are thought to be introduced to Crete.
Species |
Original range |
Comments |
Rumina decollata (LINNÉ 1758) |
Northern Africa (?) |
Today widespread in eastern Crete, but lives also at some distinct places in central and western Crete. Most habitats in Crete are in coastal areas. Also introduced to Día, Psíra, Agios Nikólaos island near Móchlos, Koufonísi, Gianisáda, Dragonáda, Prásonisi (Dionisádes), Elása, Strongiló near Koufonísi, and Pondikonísi. The species belongs to a tropical family with African affinities, Rumina being its single European representative. Was also brought to North America. |
Limacus flavus (LINNÉ 1758) |
(southern Europe) |
Synanthropic species, today widespread not only in Europe. Its original range is difficult to define exactly, somewhere in southern Europe (WIKTOR et al. 1994). |
Deroceras oertzeni (SIMROTH 1889) |
Cyclades (Andros etc.) |
The slug occurs on several islands of the Cyclades and was introduced near Agios Nikólaos, eastern Crete (WIKTOR et al. 1994). |
Oxychilus camelinus (BOURGUIGNAT 1852) |
Border area between Turkey and NW Syria |
Was introduced to a few localities in other eastern Mediterranean regions. In Crete it was found only in Chaniá and Iráklio (RIEDEL 1992). |
Oxychilus cyprius (PFEIFFER 1847) |
S Balkans and S Asia Minor |
Tends to be synanthropic in southern Greece, the Aegean islands and Crete. In Crete it was found only in Iráklio, Soúda and Chaniá (RIEDEL 1992). |
Xerotricha conspurcata (DRAPARNAUD 1801) |
Iberian peninsula (?) |
Was introduced to many places in the eastern Mediterranean (HAUSDORF 1990). In Crete it was discovered only at one locality, near Knosós. |
Xeropicta krynickii (KRYNICKI 1833) |
From the Black Sea region to Azerbaidzhan and Iran. |
At present common and widespread in the eastern Mediterranean from the Black Sea to Egypt (FALKNER 1990). Also introduced to Grándes, Gavdopoúla and Gávdos. |
Xeromunda candiota (MOUSSON 1854) |
Southern Italy |
Today the species is common in the eastern Mediterranean and northern Africa (FALKNER 1990). Widespread in western and central Crete. Also introduced to Gávdos. |
Microxeromagna armillata (LOWE 1852) |
Iberian peninsula or NW Africa (?) |
Was brought to many localities in the eastern Mediterranean (HAUSDORF 1990). In Crete it was found only at one locality in western Crete (Akra Spátha). |
Trochoidea pyramidata (DRAPARNAUD 1805) |
Southern Italy or Sicily (?) |
Lives at a number of distinct localities in the eastern Mediterranean. For Crete it has not been reported before, but it was discovered (anatomically determined by B. HAUSDORF) in 1996 at 5-9 km east of Ierápetra in the coastal areas (UTM LU9474-LU9073), and at 1-2 km west of Paleochóra near the coast (GE4202). |
Cochlicella acuta (MÜLLER 1774) |
Canary islands, NW Africa or Iberian peninsula (?) |
The species is expanding its area in the eastern Mediterranean and at the Turkish Black Sea coast (HUDEC 1973, FALKNER 1990). In Crete it is common along beaches, but is also found at synanthropic localities in the interior. Also introduced to Kaváli (E island), Koufonísi, Tráchilos near Koufonísi and Gávdos. |
Caracollina lenticula (MICHAUD 1831) |
Iberian peninsula (?) |
Lives at a few localities in coastal regions of central and eastern Crete. Also introduced to Koufonísi, Marmára and Tráchilos near Koufonísi, Chrisí, Agios Nikólaos near Móchlos, and Gávdos. |
Theba pisana (MÜLLER 1774) |
Morocco |
Has been widespread in regions of Mediterranean climate (GITTENBERGER & RIPKEN 1987). Edible species of local importance in Crete. Also introduced to Koufonísi, Chrisí and Mikronísi, Gavdopoúla and Gávdos. |
Helix aperta BORN 1778 |
Italy (?) |
Edible species, but not commercially exploited. At least 16 local names are given to this snail in Crete (Fig. 1). |
Helix nucula PFEIFFER 1859 |
Egypt, Libya |
Established populations live in Chrisí and at 5 distinct areas at the southern coast of Crete, an extinct population was discovered in Koufonísi (WELTER-SCHULTES & WIESE 1997). Edible species, only of local importance. |
Helix aspersa MÜLLER 1774 |
Italy |
Edible species, in Crete commonly sold on markets and exported to France (factory in Dafnés, near Iráklio). Also introduced to Día, Koufonísi, Chrisí and Gávdos. The species was also introduced to other continents. |
Some species of Mediterranean land snails dispersed over large distances
in pre-historic times. Listing the original ranges of Theba, Rumina, and many
Hygromiidae neither does imply that these species were dispersed by humans from
these original regions throughout the Mediterranean, nor does it allow to conclude
for commercial relations of Crete with these regions.
For the other Aegean islands, this list can be applied without Deroceras oertzeni,
and the species Ferussacia folliculus, Tandonia totevi, Deroceras panormitanum,
Monacha cartusiana and eventually Eobania vermiculata should be added to the
list.
Proportion of introduced snails in Crete
In the Cyclades the proportion of introduced snails varies between 30 and 65 % (MYLONAS 1982). In Crete this proportion is smaller. For the total number of Cretan snail species, approximately 15-20 species should be added to the lists of 112 and 114 species presented in VARDINOYANNIS (1994, different pages), where relatively low species numbers were considered for Mastus (4 species), Orculidae (2 species) and Xerocrassa (3 species). The statements referring to these groups would have required separate studies. They also lack completely (i) any morphological or anatomical diagnosis of the so-called species and (ii) any reference or explanation for the obviously arbitrary choice of old taxa. For Mastus, 13 species are listed in MAASSEN (1995). 6 Orculidae species are believed to live in Crete (E. GITTENBERGER, personal communication, 1997). As a result of these reflections, the estimated number of autochthonous land snails in Crete is 120 ± 10 species. Consequently the proportion of introduced snails in Crete (12 %) is considerably smaller than in the Cyclades. For the small islands around Crete, larger proportions of introduced snails are observed for Tráchilos near Koufonísi (44 %), Koufonísi (43 %), Mikronísi near Chrisí (43 %), Chrisí (33 %), the Kaváli islands (33 %), Pondikonísi (33 %), Grándes (30 %) and Gávdos (27 %), whereas in Día (18 %), Psíra (17 %), Dragonáda (10 %), the Paximádia islands (8 %), Gavdopoúla (8 %) and Gianisáda (7 %) the proportions are smaller than in the Cyclades.
Plausible ways of human-based land snail dispersal
The 16 species introduced to Crete are either edible, synanthropic or live
at beaches.
1 - Edible snails. Edible snails such as Helix were dispersed by humans deliberately.
In Crete, Helix aspersa and Eobania vermiculata (MÜLLER 1774) are commonly
sold in the regional markets. Helix aperta is collected in all regions of Crete,
but only in small numbers without being commercially exploited. In some regions,
Helix nucula and Helix cincta MÜLLER 1774 are also consumed. Theba pisana
and Xerocrassa cretica are consumed in parts of the island, they can also be
found on some markets.
The different local Cretan names of Helix aperta indicate (WELTER-SCHULTES,
submitted) that the edible species was transported by humans from Italy to Crete
in the Roman period (between 67 BC and 395 AD). The results of an area linguistic
study effected in 1996 (Fig. 1, own results) suggest that the Romans also brought
its name to Crete, which derived from the latin root murmur (referring to the
sound produced by the animal when lifted from the soil). From there it seems
to have spread to other regions of Crete, frequently submitted to alterations
of the foreign name. The fact that the names in the region south of Díkti
(Viános) and in eastern Crete refer to christian priests' or nuns' black
clothing (which is compared with the colour of the meat of this particular snail)
suggests that H. aperta reached these regions after 600 AD.
Establishing populations of edible snails as a form of deliberate translocation
of snail populations is not uncommon today too. Local inhabitants of Chrisoskalítisas
(southwestern coast of Crete) told me that they unsuccessfully tried to establish
a locally occurring Helix nucula population from Chrisoskalítisas at
another place at the northern coast of western Crete, near Kolimbári.
The snails layed eggs, the young hatched but did not survive. G. SERVOUDAKIS
from Alíkambos (northwestern Crete) told me that he successfully initiated
a Helix cincta population, originally from Alíkambos, on his real estate
in Attikí. He also told me that SKARIS GERMANAKIS from Léndas,
a southern Asteroúsia village, intented to settle 300 individuals of
Helix nucula from Léndas near the 70 km distant village of Alíkambos
(northwestern Crete). It was observed that the animals layed eggs in 15 cm depth,
but the young perished and the population died. Some inhabitants of the Mesará
plain and the Asteroúsia mountains also know that H. nucula from Léndas
does not survive at the northern coast of Crete. Probably the colder conditions
at the northern coast of Crete does not fit the ecological requirements of the
African snail.
2 - Synanthropic species. Snails living in cultivated fields are relatively
likely to be dispersed by humans accidentally. Eobania vermiculata, Helix aperta,
Xeromunda candiota, Xeropicta krynickii and Xerocrassa cretica belong to these
species. They are transported with harvest products (fruits, straw etc.) within
Crete and abroad. In 1996 I saw a farmer near Sitía in eastern Crete
accidentally transporting Xerocrassa cretica and Eobania vermiculata in his
pickup truck together with agricultural products. Mediterranean snails are frequently
found in central European fruit markets. For instance, Xerosecta oranensis from
Northern Africa, Eobania vermiculata and Helix aperta were introduced with fruits
and vegetables to markets in Schleswig-Holstein, Germany (WIESE 1991). Other
synanthropic species live close to human settlements, and were spread all over
Europe in early historical times. Limacus flavus, Oxychilus camelinus, Oxychilus
cyprius and Xerotricha conspurcata belong to this group. The Oxychilus populations
of the "young" cities Iráklio and Soúda must have been
introduced relatively recently (last 1000 years), while X. conspurcata is likely
to have been introduced before the destruction of the palace of Knosós
around 1400 BC.
3 - Beach species. Some snails live at beaches and may crawl into fishermen’s
boats pulled ashore. Once these boats are pulled ashore at another beach, the
snails can initiate a new population. This is a probable option for the dispersal
of Theba pisana, Cochlicella acuta, Caracollina lenticola, Trochoidea pyramidata
and Rumina decollata.
Plausible ways of transport for Albinaria
The Cretan Albinaria is a group of endemic clausiliid snails (more than 20
species, WELTER-SCHULTES 1998), the distribution patterns of which are difficult
to understand. Studies of morphological and molecular genetical features are
frequently contradictive and provide obstacles for phylogeographic conclusions.
Albinaria transportation by birds or mammals may be postulated, but no data
exist on the probability of this type of dispersal. Dispersal by humans in a
densely populated area such as Crete in Minoan and Roman times is a possible
option for exposed living snails. However, scenarios of human-based Albinaria
dispersal must be plausible.
Albinaria is too small to be eaten by humans. It does not live in agricultural
crops, and feeding on algae of calcareous rocks, it does not consume any agricultural
product. The genus is totally absent in large areas south and southwest of Iráklio
(WELTER-SCHULTES 1998), an agriculturally intensively occupied region. Albinaria
is also missing in plains without rocks, such as the Mesará, the Timbáki
plain, the Pediádas plain and the Oropédio Lasithíou, which
in turn are the most important agricultural centres of central Crete. These
observations suggest that high agricultural impact in a region results in lower
Albinaria population densities. Consequently, Albinaria is relatively unlikely
to be dispersed together with agricultural products.
The rock-dwelling Albinaria is rare on beaches, it does not live in pure sandy
areas. At some beaches however, boats may be parked close to Albinaria-inhabited
rocks, eventually providing a possibility for the snails to crawl into boats.
In order to successfully disperse Albinaria, this situation (boats being parked
close to rocks) must occur not only at the start of the boat trajectory, but
also at the locality of destination, a constellation which is unlikely to happen
frequently. The fact that boats are made of wooden material instead of rock
further reduces the probability for successful accidental Albinaria dispersal
on boats.
We may conclude that Albinaria is relatively unlikely to be transported by usual
means of land snail dispersal in the eastern Mediterranean. This conforms with
the observations (i) that only once in the history, was a Cretan Albinaria successfully
introduced to any other place outside Crete (A. cretensis from western Crete
to Lésvos (NORDSIECK 1977, BANK 1988)), and (ii) that no other clausiliid
snail has ever been successfully introduced to Crete.
However, Albinaria could be transported within an island from one place to another.
Local population numbers of some particular Albinaria species are large, especially
in areas of calcareous rocks. Large quantities of stones were needed to build
up palaces and cities like Knosós (Minoan period) and Górtis (Hellenic
and Roman period), providing plausible opportunities for artificial Albinaria
dispersal. Also in the Byzantine and Venetian epochs, relativley large numbers
of buildings were constructed, whereas in the peroids of Arabian and Turkish
occupations, constructions were limited to a small scale, but transportation
of stones by patriots in the mountains could have occured. The most recent construction
boom in Crete, which started in the 1970s, probably has not had very much influence
on Albinaria dispersal as the material needed for roads and hundreds of hotels
is not taken directly from quarries as in the centuries before.
Recognizing human-dispersed Albinaria populations
Albinaria shows characteristic distribution features in central Crete, with
each species occupying a particular and well-defined area (WELTER-SCHULTES 1998).
It is believed that the snails lived on the Cretan Neogene paleoislands between
the lower Tortonian (11-10 Ma) and the Upper Pliocene (3-2 Ma), and that the
Neogene terrain which then emerged after the uprising of Crete around the Pliocene-Pleistocene
boundary, was settled by particular species (named as group 2, consisting of
particular taxa: A. cretensis, A. terebra, A. corrugata inflata, A. praeclara
parallelifera, A. teres) (WELTER-SCHULTES 1997). Other species (group 1, other
species or subspecies: A. idaea, A. corrugata corrugata, and others) did not
migrate into the Neogene areas.
Several populations occupying ranges which do not conform with this theory are
conspicious and human-based dispersal could be suspected. I analysed such populations
in central Crete and found that they fit several other conspicious conditions
strongly supporting this idea.
A - punctual distribution. The settled area does not extend over several
km2.
B - relatively short distance (less than 10 km) to the next area inhabited by
the same subspecies.
C - isolated population. Distribution area forms an island in the compact range
of another species.
D - locality near an old city or village, or at a frequently travelled old route.
E - population of group 2 snails, which live more sun-exposed and prefer more
open habitats, not crevices.
F - morphology of population conforms with particular populations of another
occupied area of the same species.
G - plausibility that stones might have been transported.
H - group 2 snails on preneogene surfaces, or group 1 snails on Neogene surfaces.
Conditions A-D are expected features of human-dispersed populations. Snails living in more open habitats (exposed at the face of the rocks, E) are more likely to be dispersed by humans than species living in crevices and under the rocks. In some cases (A. idaea, A. hippolyti) it is possible to determine with a high plausibility by analysis of the shell morphology from which region the dispersed population probably could have come from (F). The probability of human Albinaria dispersal within Crete is enhanced in situations where big natural calcareous stones or rocks could have been transported (G). The population's geomorphological surface (Neogene, preneogene) being different from that of all other populations of the same taxon also indicates human-based dispersal (H).
Examples of human-dispersed Albinaria populations
Several central Cretan populations fitting to condition A were analysed. Here I list the cases which responded positively to at least two other conditions. In all presented cases anthropochorous dispersal is highly probable.
1 - near Zarós: A. corrugata inflata (Fig. 2A)
A, B, C, D, E, H
One small A. corrugata inflata population (group 2) lives in (UTM) LU0791 (3
km NW Zarós, the geographic positions of the localities are shown in
Fig. 1) close to the Agios Andónios monastery, this area forms an island
in a complex area inhabited by A. idaea, A. hippolyti and A. terebra. The isolated
A. c. inflata population is located at the road from Agía Varvára
via Zarós to Kamáres, an old and frequently travelled road at
the southern slopes of the Idi mountains. It is at 5-6 km distance west of the
nearest point of the compact range of A. c. inflata. Stones could have been
transported along the road to build the monastery. The isolated population lives
on preneogene terrain, whereas the other populations of the subspecies A. c.
inflata nearly exclusively live on Neogene surfaces.
2 - near Krousónas: A. idaea (Fig. 2B)
A, C, D, (F), G
A small isolated A. idaea population (group 1) lives in LV1202, at 5-6 km NW
of Krousónas in the uninhabited mountains of Idi at 1000 m a.s.l.. The
population lives in the compact range of A. hippolyti (Fig. 2C). The locality
is at a distance to the compact A. idaea area of 8-9 km to the southwest, and
of 17-18 km to the west. It is situated along the road from Krousónas
to Anógia. This road is not frequently travelled today, but another situation
can be suggested in times when many Cretans were forced to live in the mountain
regions of the island (periods of Arabian, Venetian, Turkish or German occupations).
Stones could have been transported for different purposes (mitáto constructions,
constructions of shelters for patriots, etc.). The measurements presented in
ENGELHARD & SLIK (1994) (especially the height of the parietalis, which
seems to be submitted to regional variation rather than altitude dependent,
results of own studies) suggest that the population in LV1202 is closer related
to the A. idaea populations northwest of the Psiloríti peak than to the
ones near the Idéon Andron cave (Fig. 2B). Further analyses are necessary
to strengthen this hypothesis. The locality is on preneogene terrain.
3 - Górtis: A. corrugata inflata (Fig. 2A)
A, B, C, D, E, G
A. corrugata inflata populations (group 2) around LU1281 (2-4 km E-W extension)
live in an area of 2-5 km2. This territory forms an island in the several hundred
km2 sized compact range of A. terebra. The isolated A. c. inflata terrain is
at 4-6 km distance south of the nearest point of the compact range of A. c.
inflata. LU1281 corresponds with the position of the ancient city of Górtis,
the most important settlement in the Roman period. A. c. inflata lives on the
ruins. At some places, A. terebra and A. c. inflata were found living sympatrically.
It is likely that large quantities of stones were carried from the surrounding
regions north of the Roman city for the construction of buildings, and that
A. c. inflata was brought to Górtis between 800 BC and 800 AD with construction
material. The city was destroyed in 824 AD. It extended approximately 2-3 km
from LU1281 to the south. These terrains are in the Mesará plain which
is not inhabited by any Albinaria species, so A. c. inflata could not have dispersed
southwards. The isolated populations live on Neogene and Quaternary terrain,
primarily on the ruins and on rocks close to the ruins.
4 - Ano Moúlia: A. hippolyti holtzi (Fig. 2C)
A, B, C, D, F, H
The A. hippolyti holtzi (group 1) population lives in LU1788, near Ano Moúlia
(SCHILTHUIZEN et al. 1993). The area forms an island in the compact range of
A. terebra. Distance to the compact A. hi. holtzi terrain in the northwest is
4-5 km. The isolated population lives on Neogene (Tortonian) terrain, all other
A. hi. holtzi populations live on preneogene surfaces. A late immigration of
A. hi. holtzi without human influence cannot be excluded, however, human-based
dispersal seems to be the more probable option.
5 - Káto Moúlia: A. hippolyti aphrodite (Fig. 2C)
A, C, D, E, F, G
A population of A. hippolyti aphrodite (group 2) lives in LU1785-LU1786, on
a hill west of Káto Moúlia. The isolated population lives on Neogene
(Tortonian) terrain. The area forms an island in the compact range of A. terebra.
The area around the hill west of Káto Moúlia is surrounded by
cultivated fields, a constellation which prevents the snails from leaving the
hill. It is possible to determine the original range of the Káto Moúlia
population by analysis of its shell morphology. The population with dorsally
to dorsolaterally situated lunula exhibits a strongly developed frontal upper
palatal fold (FUP, see SCHILTHUIZEN et al. 1993) in 38 % of 45 examined specimens,
weakly developed in 53 % and absent in 9 %. The only population groups with
variable FUP are (A) Agios Míronas-Loutráki-Krousónas,
(B) Profítis Ilías-Agios Sílas, and (C) Amourgéles.
The three candidate populations are distinguished by the proportion of strongly
developed to absent FUP. In population A the FUP is strongly developed only
in 12 %, but absent in 60 % of n = 62 specimens, in B the relation is 55 % strongly
developed and 20 % absent (n = 177), in C it is 85 % strongly developed and
2 % absent (n = 47). Given 2 points per percent strongly developed FUP, 1 point
for weakly developed FUP, and 0 points for absent FUP, we arrive at a FUP factor
of 129 for Káto Moúlia, 52 for A, 135 for B and 183 for C. The
factor for Káto Moúlia fits well with the value for population
B, but not with the other two populations. The results suggest that the Káto
Moúlia population derived from the region Profítis Ilías-Agios
Sílas, at 13-14 km distance from Káto Moúlia. Stones could
have been transported from an ancient quarry (at 2 km south of Profítis
Ilías, LU2796) to Górtis. Both villages Profítis Ilías
and Káto Moúlia are along the most direct route from Knosós
to Górtis and Festós, probably a frequently travelled path in
Minoan and Roman times. Agios Sílas was settled in the Minoan period
(TOUCHAIS 1986, TOUCHAIS 1987). The site of Lykastos is in the same area. Lykastos
was mentioned by Homer around 800 BC (Il. B, 647), but also afterwards it had
connections to Knosós and Górtis in the Hellenistic period. However,
the artificial dispersal could also have taken place in the epochs after, when
the region continued to be densely settled.
6 - Veneráto-Kipárisos: A. hippolyti holtzi (Fig. 2C)
A, B, C, D, F, H
In LU2296 (1.5 km E Veneráto, along the road to Kipárisos), a
single specimen of A. hippolyti holtzi (group 1) was found in 1990. The position
is at 5.5 km distance to the compact A. hi. holtzi range of Idi. The specimen
was found on Neogene terrain, while the natural habitat of A. hi. holtzi consists
exclusively of preneogene rocks. The locality is in the compact range of A.
corrugata inflata (group 2, Fig. 2A), but its population density is extremely
low in the region where A. hi. holtzi was found. I am not sure whether the A.
hi. holtzi population is stable. The determination as A. hi. holtzi is reliable
(lateral lunula, FUP absent, basalis present but weak, SCHILTHUIZEN et al. 1993).
The subcolumellaris is weakly developed (it is strongly developed in most of
the southern populations of A. hi. holtzi), the subcolumellaris invisible from
the aperture, suturalis absent. These features and the weakly developed basalis
suggest that the population could have been brought from near Asítes
(around LU1596), on the road via Veneráto. The area between Veneráto
and Profítis Ilías is densely occupied by agricultural activities,
including terraces for olives. The involved road could be very old, Kipárisos
was settled in Minoan times (PARIENTE 1992). The artificial dispersal could
have taken place in any epoch since then.
7 - Knosós: A. praeclara parallelifera (Fig. 3A)
(A), B, C, D, E, G
A. praeclara parallelifera populations (group 2) around LV3406-LV3407 (2-3 km
extension in all directions) live in an area of 10-15 km2, which forms an island
in the compact range of A. corrugata inflata (Fig. 2A). The isolated A. pr.
parallelifera terrain is at 4-5 km distance west of the nearest point of the
large area inhabited by A. pr. parallelifera. The isolated A. pr. parallelifera
area corresponds with the position of the Minoan palace of Knosós (the
palace ruins are in LV3207-LV3307), the most important settlement in Minoan
periods. At some places, both species were found living sympatrically. It is
likely that large quantities of stones were transported from the surrounding
regions to Knosós for the construction of the palace, and that A. pr.
parallelifera has been brought between 2000 and 1500 BC with construction material
to the Minoan palace. If so, then the locality of introduction is at a distance
of 7 km to the compact range of A. pr. parallelifera in the east. The isolated
population lives on Neogene terrain.
8 - Ligórtinos: A. praeclara parallelifera (Fig. 3A)
A, B, C, D, E, G
A very conspicious population of A. preaclara parallelifera (group 2) lives
on rocks on the hill of the village of Ligórtinos (LU3482). This locality
forms an island in the compact range of A. teres (group 2, Fig. 3C). The isolated
A. pr. parallelifera terrain is at 3 km distance south of the nearest point
of the compact range of A. pr. parallelifera. The position of the exposed hill
of the old village strongly suggests human introduction of the snail. Today
the hill is used for goat rearing. Stones could have been brought from nearby
regions to the place to build shelters or primitive buildings. The locality
is on Neogene terrain. As the hill is between the village and agricultural crops,
the snail has had no possibility to disperse by its own.
9 - Lasíthi Plateau/road to Neápoli: A. praeclara praeclara
(Fig. 3A)
A, C, D, E
An A. praeclara praeclara population (group 2) lives in LU6393-LU6392, between
the village of Agios Konstandínos and the Kristalénias monastery.
Another population lives near Mésa Lasitháki, 2 km east of the
monastery population. The two populations are not connected. The settled areas
form islands in the compact range of A. corrugata corrugata (group 1). The isolated
A. p. praeclara terrain is at a distance of 17-18 km west of the nearest point
of the compact range of A. p. praeclara in the lowlands. Stones could have been
transported to the locality of the old monastery from the Agios Nikólaos
region along the Potámi valley road (via Exo Potámi), to build
the monastery or houses of the nearby villages. However, it sounds somehow strange
that natural stones should be carried from the lowlands over such long distances
to a karstic mountain region. The isolated population lives on preneogene terrain.
Human-dispersed A. maltzani populations live in the same region (see 10). A
step by step transportation of A. p. praeclara could be possible.
10 - Lasíthi Plateau/road to Neápoli: A. maltzani (Fig. 3B)
A, C, D
Along the road from the Lasíthi Plateau to Neápoli, between Mésa
Lasíthi and Mésa Potámi, live several populations of A.
maltzani (group 1, around LU6594). The settled areas form islands in the compact
range of A. corrugata corrugata (group 1). The isolated A. maltzani terrain
at 1000 m a.s.l. is at a distance of 7-8 km west of the nearest A. maltzani
populations near Zénia and Amigdáli, and at a distance of 15-20
km southwest of the compact A. maltzani range northeast of Neápoli. The
isolated populations live on preneogene terrain. Possibly human-dispersed A.
praeclara populations live in the same region (see 9).
11 - Zénia/Amigdáli: A. maltzani (Fig. 3B)
A, B, C, D
The A. maltzani populations near Zénia and Amigdáli (around LU7396)
live close to the Potámi valley road from Neápoli to the Lasíthi
Plateau (see 9 and 10). Their area is isolated in a compact A. corrugata corrugata
range, their distance to the compact A. maltzani range in the north is 4-5 km.
The isolated populations live on preneogene terrain.
12 - Mílatos cave: A. corrugata inflata (Fig. 4A)
A, B, C, D, E, G
A small population of A. corrugata inflata lives in the close vicinity of the
Mílatos cave (LV7107). The small area is located in the compact range
of A. hippolyti arthuriana with which A. c. inflata lives syntopically. The
isolated population is at a distance of 2-3 km west of the nearest point of
the compact range of A. c. inflata between Mália and the village of Mílatos.
The cave is famous because of the Mílatos cave massacre with several
thousand victims which took place in 1823. In the last decades a modern footpath
was constructed which connects the modern road (Mílatos-Agios Andónios)
with the cave. The stones which were used to construct the path were not taken
from the close vicinity but obviously from another region, probably from near
an area southwest of the village of Mílatos, 3-4 km west of the cave.
It is possible that A. c. inflata was artificially dispersed together with the
stones.
13 - Día: A. teres teres (Fig. 3C, 4B)
D, F, G
A. teres teres lives in a large area on the island of Día, but its range
conspiciously does not include the easternmost parts of the island (WELTER-SCHULTES
1992). The position of the eastern range limit on Día is shown in Fig.
4B. This borderline does not correspond to any natural borderline in the sense
of different ecological conditions prevailing at both sides of it. Albinaria
teres occurs in eastern Crete (Sitía peninsula) on both preneogene and
Neogene rocks. West of this peninsula, A. teres is exclusively found on Neogene
surfaces southeast, south and southwest of Díkti, and along some southern
slopes of the Asteroúsia mountains. These features indicate that the
original prepliocene range of A. teres was located on the Sitía peninsula.
The subspecies A. t. teres occurs at Akra Síderos (SCHULTES & WIESE
1991), a strategically important cape of northeastern Crete (occupied by the
Greek Navy today). It is well-known that this cape was frequently used by pirates.
Sailing from Akra Síderos in order to attack Chandax (= Candia, Iráklio),
pirates of the 10th century could have enhanced the weight of their ships with
big stones (taken from Akra Síderos) in order to avoid to be seen from
the coast. They could have approached Chandax from the north, hiding at the
farside of the island of Día (Fig. 3C). There they could have seeked
shelter for some days in order to wait for better sailing weather, unloading
the weight stones at the northern coast of Día, perhaps in order to build
a shelter. The most probable landing place and the dispersal directions are
shown in Fig. 4B. The access to other places at the northern coast of Día
is extremely difficult. This scenario fits approximately to the 10th and 11th
century (not earlier than 961 AD, when the city (Rabd-el-Kandak = Iráklio)
belonged to the pirates themselves, but not much later than 1150-1200 AD, when
the city was better defended). If true, then A. teres dispersed over 2.1-2.4
km indicating a dispersal velocity of 2.0-2.5 m yr-1. It would be interesting
to observe the dispersal velocity of A. teres in the remaining eastern parts
of Día during the following decades or centuries. If the species will
not extend its range to the east, the theory of human-based dispersal must be
questioned.
Apart from A. teres, Día hosts no other non-decolled Albinaria species.
The geomorphological surface conditions on Día are very similar to those
of Akra Síderos: high density of calcareous preneogene rocks, no human
impact in the form of agriculture or roads. Thus the dispersal conditions for
the introduced species were optimal.
The theory could be evaluated by analysis of molecular genetical distances between
the populations of Akra Sideros and Día, which should be relatively small.
There are no significant morphological differences between the involved populations.
A taxonomic implication is that the taxon A. teres phalanga (O. BOETTGER 1883)
described from Akra Síderos is considered as a junior synonym of A. teres
teres (OLIVIER 1801) descibed from Día.
Dispersal velocity of Albinaria
Based on the observations of Albinaria teres on Dia, and if the here presented
plausible theory (dispersal by pirates in the 10th-11th century) is correct,
the maximum dispersal velocity of Albinaria under optimal conditions (habitat
of densely dispersed calcareous rocks, absence of any human impact and competing
species) does not exceed 2.5 m yr-1. This more or less conforms with previous
estimations (1-2 m yr-1 in SCHILTHUIZEN & LOMBAERTS 1994).
This hypothesis is strengthened by the plausible dispersal scenario for the
A. praeclara parallelifera population at Knosós palace. It is likely
that the snail was brought to the palace around 2000-1500 BC (Minoan period,
when the palace was built). Since then it would have dispersed 1 km to the south,
2.5 km to the southeast, 2 km to the east, 1 km to the northeast, and probably
0-0.5 km to the west. Taking the maximum distance of 2.5 km (Knosós to
the southeast) and 4500 yr of dispersion results in a mean dispersal velocity
of 0.5-0.6 m yr-1. In contrast to the situation of A. teres on the island of
Día, A. pr. parallelifera had a competing species (A. corrugata inflata).
This explains the generally lower dispersal velocity at Knosós when compared
with Día. There is also considerable agricultural impact in the area
around Knosós. The uneven distribution of the dispersal velocity towards
different directions is most probably related to the high agricultural impact
present in the areas where the dispersal velocity resulted to be very low.
Discussion
Because artificial dispersal of Albinaria has never been documented the method
of identifying human-dispersed populations must rather be stochastic than deterministic.
The presence of particular conditions provide high probabilities that a particular
population originated through human dispersal. In fact, a small extension of
the area settled by the questioned population (A), and the vicinity of an ancient
city, old villages or old roads (D) were observed in all conspicious cases in
Crete. However, the island was a densely populated island in Minoan and Roman
times, and there are few regions far from ancient settlements, paths or roads.
A and D taken alone are unlikely to provide deterministic evidence for anthropochorous
dispersal. This is why additional factors need to be considered (B, C, E-H).
One of these factors is the eventual vicinity of a compact distribution area
of the same subspecies, from which the trajectory of the questioned population
is thought to have started (B). The observed trajectory distances in Crete vary
between 3 and 20 km (average = 8.7 km).
The presented method allows us to recognize only those kinds of human-dispersed
populations were species were brought to compact ranges of other Albinaria species
(C). If snails are transported within a particular species' range from point
A to another point B, then the artificially dispersed snails from point would
breed with the existing populations at B resulting in a mixed population, so
this kind of dispersal will unlikely be detected with the here presented method.
Conclusions
The results suggest that in 12 cases Albinaria was dispersed by human activity
within central Crete over small distances from one place to another, successfully
initiating new populations at the destination localities. In two observed cases
Cretan Albinaria were transported oversea: from near Chaniá (western
Crete) to Lésvos, and from Akra Síderos (eastern Crete) to Día.
Although the dispersal of many of the artificially initiated populations is
assumed to have taken place in the Minoic, Hellenic and the Roman period, in
some populations human-based dispersal seems to have taken place in the epochs
after.
The dispersal velocity of Albinaria does not exceed 2.5 m yr-1 under optimal
conditions, but presence of a competing species will reduce the dispersal velocity
of a human-dispersed species to less than 0.6 m yr-1. If these conclusions are
correct, then Cretan populations which occupy areas larger than 10 km in extension,
are definitely to be seen as natural populations. Having found only 12 populations
in central Crete which certainly fit the presented conditions to recognize human-based
dispersal, I conclude that although some Albinaria populations were dispersed
artificially, most Albinaria populations are natural and their ranges have to
be explained otherwise.
Acknowledgements. I thank B. Hausdorf, G. Kotoulas, R. Lecanidou and E. Zouros for helpful information and discussions, and the E. Zouros lab for particular help during the fieldwork in 1996. F. Missirlis helped translating the Greek passages. This study was supported by a doctoral fellowship from the Evangelisches Studienwerk Villigst.
Peri/lhyh
Xersai/a saligka/ria e/xoun suxna/ metaferqei/
me thn anqrw/pinh drasthrio/thta sta nh/sia tou Aigai/ou. Toula/xistan 16 ei/dh
saligkariw/n eish/xqhkan sthn Krh/th apo/ dia/forej perioxe/j thj Mesogei/ou.
Ta perisso/tera e/ftasan sthn Krh/th kata/ thn arxaio/thta. Ektoj apo/ to Helix
pou trw/getai apo/ touj anqrw/pouj, ta neoferme/na saligka/ria epoi/khsan to
nhsi/ me tuxai/o tro/po.
Ta perisso/tera apo/ ta 20 krhtika/ ei/dh tou ge/nouj
Albinaria den e/xoun metaferqei/ apo/ e/cw alla/ apotelou/n topika/ endhmika/
ei/dh. H diaspora/ twn plhqusmw/n touj ei/nai du/skolo na analuqei/. Mi/a apo/
tij parame/trouj pou periple/koun th shmerinh/ touj katanomh/ ei/nai h tuxai/a
dia/spora touj apo/ ton a/nqrwpo. Se auth/ th mele/th protei/noume mia me/qodo
anagnw/rishj sto pedi/o plhqusmw/n thj Albinaria, pou e/xoun texnhta/
metaferqei/. Ta apotele/smata marturou/n pw/j ta Albinaria metafero/tan mazi/ me
tij pe/trej pou xrhsimopoiou/ntan sti/j kataskeue/j kai allou/. Parousia/zoume
gewgrafike/j qe/seij o/pou te/toioi plhqysmoi/ bre/qhkan kai meleta/me thn egkata/stash
kai thn eca/plwsh/ touj. Me ba/sh thn ana/lush du/o sugkekrime/nwn plhqusmw/n
thj Albinaria sthn Knwso/ kai sto nh/so Di/a upologi/same thn me/sh
taxu/thta diaspora/j tou ge/nouj Albinaria. Se eunoi+ke/j sunqh/kej h taxu/thta diaspora/j tou
ei/douj diakume/netai apo/ 2.0 me/xri 2.5 me/tra ton xro/no, alla/ se sunqh/kej
antagwnismou/ me a/lla paro/moia ei/dh h taxu/thta elattw/netai se ligo/tero
apo/ 0.6 me/tra ton xro/no. Plhqusmoi/ thj Albinaria twn opoi/wn thn katanomh/
e/xei ephrea/sei o a/nqrwpoj ei/nai sxetika/ spa/nioi, kai den katalamba/noun
mega/lej ekta/seij. Oi perisso/teroi plhqusmoi/ ei/nai fusikoi/ kai h fulogewgrafi/a
touj pre/pei na echghqei/ me diaforetikh/ meqodologi/a.
BIBLIOGRAPHY
BANK, R. A. (1988): Die Molluskenfauna der griechischen Insel Lesbos (= Mytilini). -- Basteria 52: 61-76. Leiden.
FALKNER, G. (1990): Binnenmollusken. - In: FECHTER, R. & FALKNER, G.: Weichtiere. Europäische Meeres- und Binnenmollusken. -- Steinbachs Naturführer 10: 112-280. München.
ENGELHARD, G.H. & SLIK, J.W.F. (1994): On altitude dependent characters in Albinaria idaea (L. PFEIFFER, 1849), with a revision of the species (Gastropoda Pulmonata: Clausiliidae). -- Zoologische Mededelingen 68: 21-38. Leiden.
GITTENBERGER, E. & RIPKEN, TH. E. J. (1987): The genus Theba (Mollusca Gastropoda: Helicidae), systematics and distribution. -- Zoologische Verhandelingen 241: 3-59. Leiden.
GLAUBRECHT, M. (1993): Die Landschnecke Levantina spiriplana im Ostmediterran: Johanniter-Kreuzritter-These oder Paläogeographie? -- Natur und Museum 123: 97-114. Frankfurt am Main.
HAUSDORF, B. (1990): Über die Verbreitung von Microxeromagna armillata (LOWE 1852) und Xerotricha conspurcata (DRAPARNAUD 1801) in Griechenland und der Türkei (Gastropoda: Hygromiidae). -- Malakologische Abhandlungen 15: 55-62. Dresden.
HUDEC, V. (1973): Helicidae (Gastropoda, Pulmonata) gesammelt von der niederländischen biologischen Expedition in die Türkei in 1959. II. -- Zoologische Mededelingen 46: 231-259.
MAASSEN, W. J. M. (1995): Observations on the genus Mastus from Crete (Greece), with descriptions of twelve new species (Gastropoda Pulmonata: Buliminidae). -- Basteria 59: 31-64. Leiden.
MYLONAS, M. A. (1982): Meléti páno sti zoogeografía ke ikología ton cherséon malakíon ton Kikládon. [The zoogeography and ecology of the terrestrial molluscs of Cyclades]. -- 236 pp. Didaktorikí Diatriví [Unpublished Ph. D. Thesis], University of Athens.
MYLONAS, M. A. (1984): The influence of man: a special problem in the study of the zoogeography of terrestrial molluscs on the Aegean islands. - In: SOLEM, A. & VAN BRUGGEN, A. C.: World wide snails. Biogeographical studies on non-marine Mollusca: pp. 249-259. -- Leiden.
NORDSIECK, H. (1977): Zur Anatomie und Systematik der Clausilien, XVII. Txonomische Revision des Genus Albinaria VEST. -- Archiv für Molluskenkunde 107: 285-307. Frankfurt am Main.
PARIENTE, A. (1992): Chronique des fouilles et découvertes archéologiques en Grèce en 1991. -- Bulletin de Correspondance Hellénique 116: 833-954.
RIEDEL, A. (1992): The Zonitidae (sensu lato) (Gastropoda, Pulmonata) of Greece. -- Fauna Graeciae 5: 8 + 194 pp. Athína.
SCHILTHUIZEN, M. & LOMBAERTS, M. (1994): Population structure and levels of gene flow in the Mediterranean land snail Albinaria corrugata (Pulmonata: Clausiliidae). -- Evolution 48: 577-586.
SCHILTHUIZEN, M., WELTER-SCHULTES, F. W. & WIESE, V. (1993): A revision of the polytypic Albinaria hippolyti (Boettger, 1878) from Crete (Gastropoda Pulmonata: Clausiliidae). -- Zoologische Mededelingen 67: 137-157.
SCHULTES, W. & WIESE, V. (1991): Die Gattung Albinaria auf Kreta: VI. Über Flügelrippen-Rassen von Kreta. -- Schriften zur Malakozoologie 4: 65-75. Cismar/Ostholstein.
TOUCHAIS, G. (1986): Chronique des fouilles et découvertes archéologiques en Grèce en 1985. -- Bulletin de Correspondance Hellénique 110: 671-761.
TOUCHAIS, G. (1987): Chronique des fouilles et découvertes archéologiques en Grèce en 1986. -- Bulletin de Correspondance Hellénique 111: 519-583.
VARDINOYANNIS, K. (1994): Viogeografía ton cherséon malakíon sto nótio nisiotikó egeakó tóxo. [Biogeography of land snails inn the south Aegean island arc]. -- 318 pp. Didaktorikí Diatriví [Unpublished Ph. D. Thesis], University of Athens.
WELTER-SCHULTES, W. (1992): Notes on the taxonomy of Nisos Dia, Crete (Gastropoda: Clausiliidae). -- Biologia Gallo-hellenica 19: 55-61.
WELTER-SCHULTES, F. W. (1997): Paleogeography and Albinaria distribution in central Crete - an approach to their evolutionary history? -- Heldia 4 (5): 62-64. München.
WELTER-SCHULTES, F. W. (1998): Albinaria in central and eastern Crete: distribution map of the species (Pulmonata: Clausiliidae). -- Journal of Molluscan Studies 64: 275-279.
WELTER-SCHULTES, F. W. (submitted): The traditional Greek names of the edible land snails in today's Crete. -- Glotta, Göttingen.
WELTER-SCHULTES, F. W. & WIESE, V. (1997): Die Landschnecken der Insel Koufonísi (südlich Kreta) mit Nebeninseln. -- Schriften zur Malakozoologie 10: 80-95. Cismar/Ostholstein.
WIESE, V. (1991): Atlas der Land- und Süsswassermollusken in Schleswig-Holstein. -- 251 pp., Landesamt für Naturschutz und Landschaftspflege, Kiel.
WIKTOR, A., VARDINOYANNIS, K. & MYLONAS, M. (1994): Slugs of the Greek southern Aegean islands (Mollusca Gastropoda nuda: Milacidae, Agriolimacidae et Limacidae). -- Malakologische Abhandlungen 17: 1-36. Dresden.
Figures
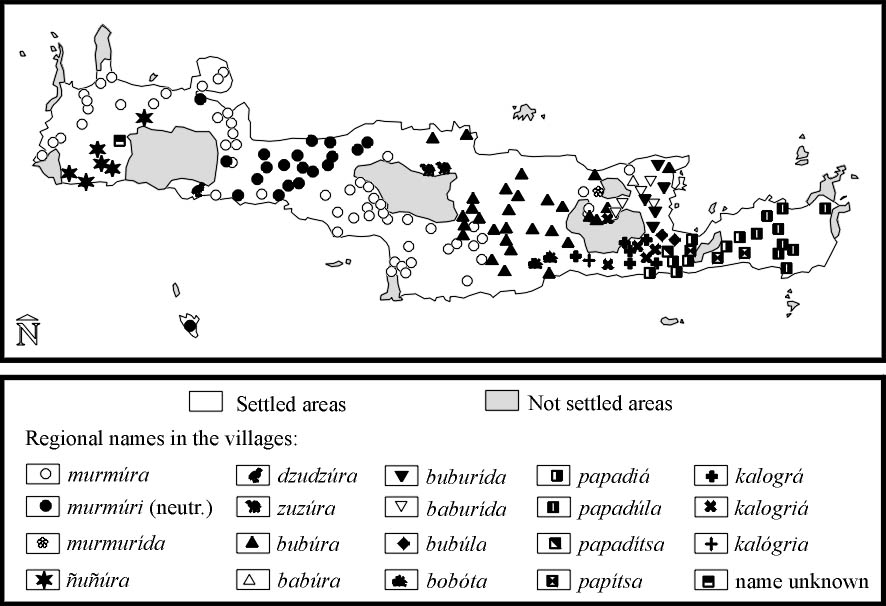
Fig. 1. Regional names for the introduced edible snail Helix aperta in Crete. The oldest name (murmúra) derived from the latin root murmur and describes the characteristic sound produced by the animal when being collected. The latin name reveals that the snail must have been introduced from a latin-speaking country (most probably Italy, in the Roman period of Crete). 11 regional alterations of murmúra reflect the history of dispersal of the snail towards all parts of Crete. In eastern Crete, a region which always kept some independence in the antiquity, the greek names papadiá and kalográ were invented by Cretans comparing the black meat of the snails with the clothings of priests and nuns.

Fig. 2A-C. Positions of artificially dispersed Albinaria populations in central
Crete, with 1000 m contours.
A - Albinaria corrugata inflata, B - Albinaria idaea, C - Albinaria hippolyti

Fig. 3A-C. Positions of artificially dispersed Albinaria populations in Crete,
with 1000 m contours.
A - Albinaria praeclara, B - Albinaria maltzani, C - Albinaria teres
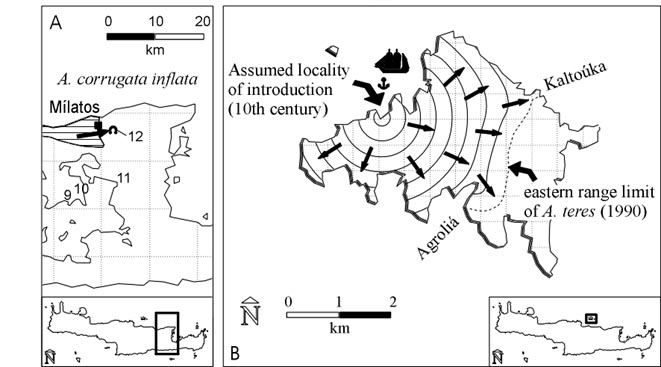
Fig. 4A. Geographic position of the artificially dispersed Albinaria corrugata
inflata population of the Mílatos cave, eastern Crete, with 1000 m contours.
The A. c. inflata population of Mílatos-Mália from which the artificially
initiated population is thought to have derived is also indicated. B. Dispersal
scenario of Albinaria teres on Día. The snail is assumed to be introduced
at the northern coast by pirates who came from Akra Síderos, eastern
Crete, around the 10th century. It is thought to have dispersed towards all
directions since then, the longest trajectory being 2.5 km to the southeast.
Address of the author:
Institut fuer Zoologie und Anthropologie der Universitaet
Berliner Str. 28
D-37073 GOETTINGEN
(Germany)
E-mail: x_fwelter@gwdg.de (leave out the x_, this is quoted to avoid spam.
Write only fwelter)